Fully π-fused propellanes: an emerging class of 3D building blocks
Use as 3D building blocks
Although biphenyl- and naphthalene-fused propellanes were first synthesized in 1971 and 1993, respectively, their use has been quite limited as compared with other 3D building blocks such as tetraphenylmethane, spirobifluorene, triptycene, and their derivatives.
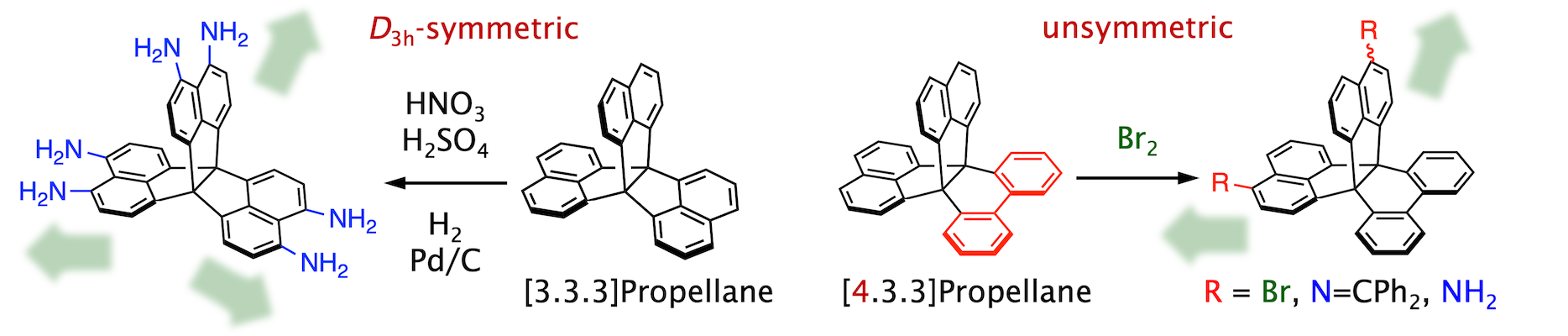
We developed new functionalization methods including D3h-symmetric hexa-nitration of [3.3.3]propellane (BCSJ 2022) and unsymmetric functionalization of [4.3.3]propellane (CC 2023).
In addition, we have gradually improved synthetic protocols of the fully π-fused propellanes toward scalable and easy ones (CEJ 2024, see SI).
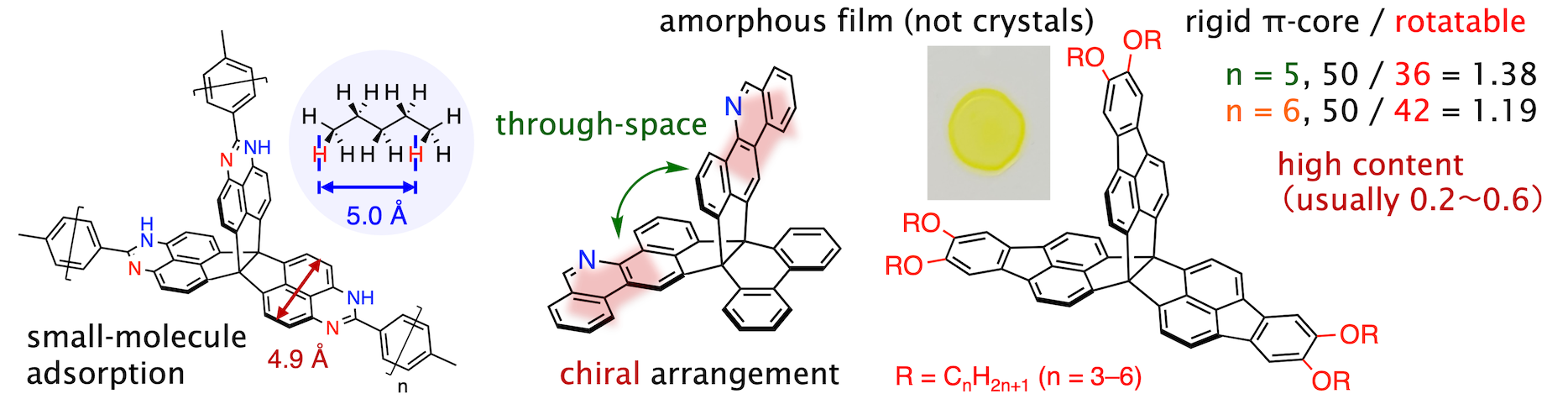
Small-molecule gases and vapors were adsorbed in polymeric and hydrogen-bonding solids of [3.3.3]propellanes (BCSJ 2022; CL 2022).
Planar chromophores were arranged on a [4.3.3]propellane for chiroptical response (CC 2023).
Twisting π-skeletons
Whereas triptycene has a bicyclo[2.2.2]octane core with external C–H bonds, propellanes have tricyclo[l.m.n.0]alkane cores with internal C–C single bonds. The difference enable twist depending on surrounding loop moieties.
We investigated the most stable conformations, transition states for twist inversion, and twisting flexibility (CEJ 2024).
A set of [3.3.3]propellanes unexpectedly gave amorphous films despite very high π-core content (CAJ 2024).
