Pillar[n]arenes: Synthesis, functionality and planar chirality
Polygons are beautiful structures with excellent symmetry. In 2008, we developed a new class of pillar-shaped macrocyclic hosts. Based on their highly symmetric pillar-shape, we named this type of paracyclophanes "pillar[n]arenes". After their discovery, many papers using pillar[n]arenes have been published (>1700). Thus, pillar[n]arenes are one of the game-changing molecules in supramolecular chemistry.
Synthesis & Structure
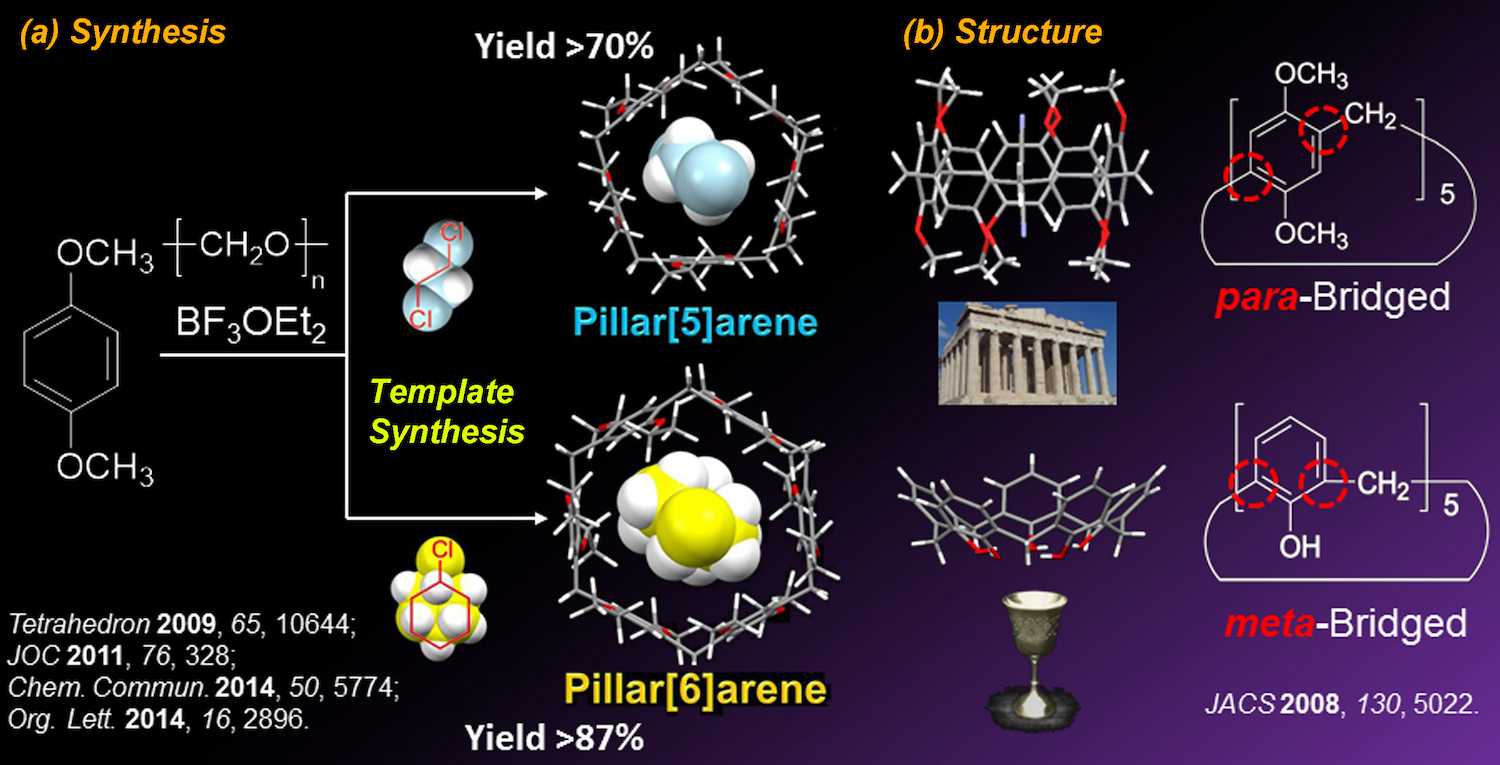
Pillar[n]arenes (n = 5, 6) can be prepared selectively depending on the solvent used for the cyclization reaction. For example, pillar[5]arenes can be synthesized in high yields using 1,2-dichloroethane as a solvent. In contrast, pillar[6]arenes can be synthesized in high yields when chlorocyclohexane is used as a solvent. In both cases, solvents work as templates to facilitate the production of a particular pillar[n]arene homologue in high yields. The regents typically used to prepare pillar[n]arenes are commercially available and inexpensive, and the products can be readily isolated using a simple recrystallization. Based on their easy synthesis, pillar[5]arene bearing 10 methoxy groups has been sold commercially by Tokyo Chemical Industry (TCI) since 2014.
The most important point regarding the chemical structures of pillar[n]arenes is the position of the methylene bridges. Pillar[n]arenes are composed of 1,4-dialkoxybenzene units, which are connected by methylene bridges at their 2- and 5-positions. In contrast, in calix[n]arenes, the phenol units are linked by methylene bridges at their 2- and 6-positions. Due to the different methylene bridge positions, the shapes of pillar[n]arenes are completely different from those of calix[n]arenes. Calix[n]arenes have open-ended, non-symmetric calix-shaped structures, thus they are named calix[n]arenes. In contrast, pillar[n]arenes have completely symmetric cylindrical structures. Based on the highly symmetric pillars, we named this type of paracyclophane "pillar[n]arenes".
Functionality
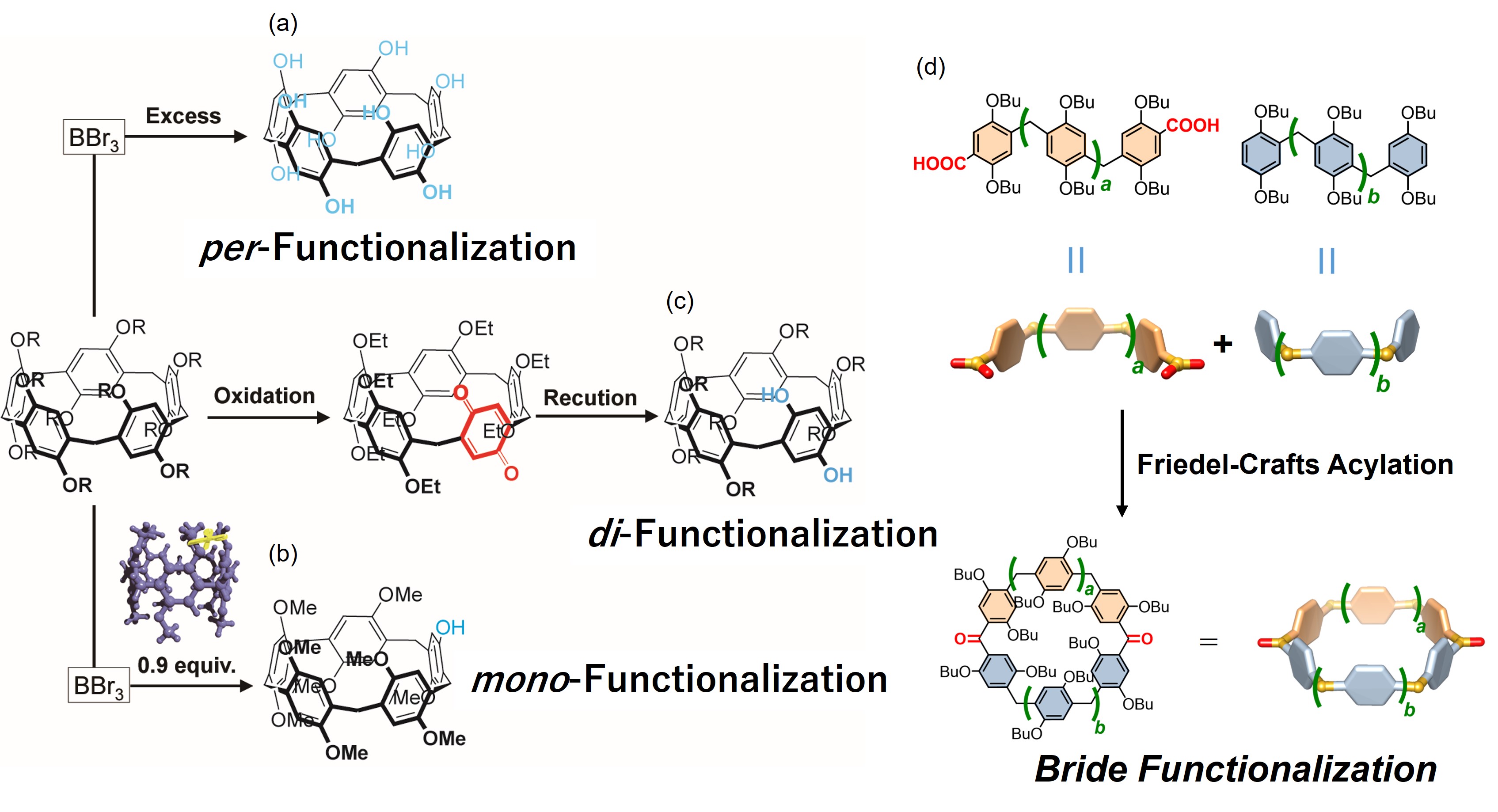
The alkoxy groups on both rims of pillar[n]arenes can be converted to highly reactive phenol groups by de-protection using BBr3. By tuning of the amount of BBr3, we can access pillar[n]arenes with mono- and per-phenol groups. Pillar[n]arenes with two phenol groups in the same unit can be accessed by oxidation/reduction of one dialkoxybenzene unit. By utilizing the high reactivity of the phenol groups, we are able to produce pillar[n]arenes with various substituents. Recently, bridge functionalization can be possible by Fridel-crafts acylation.
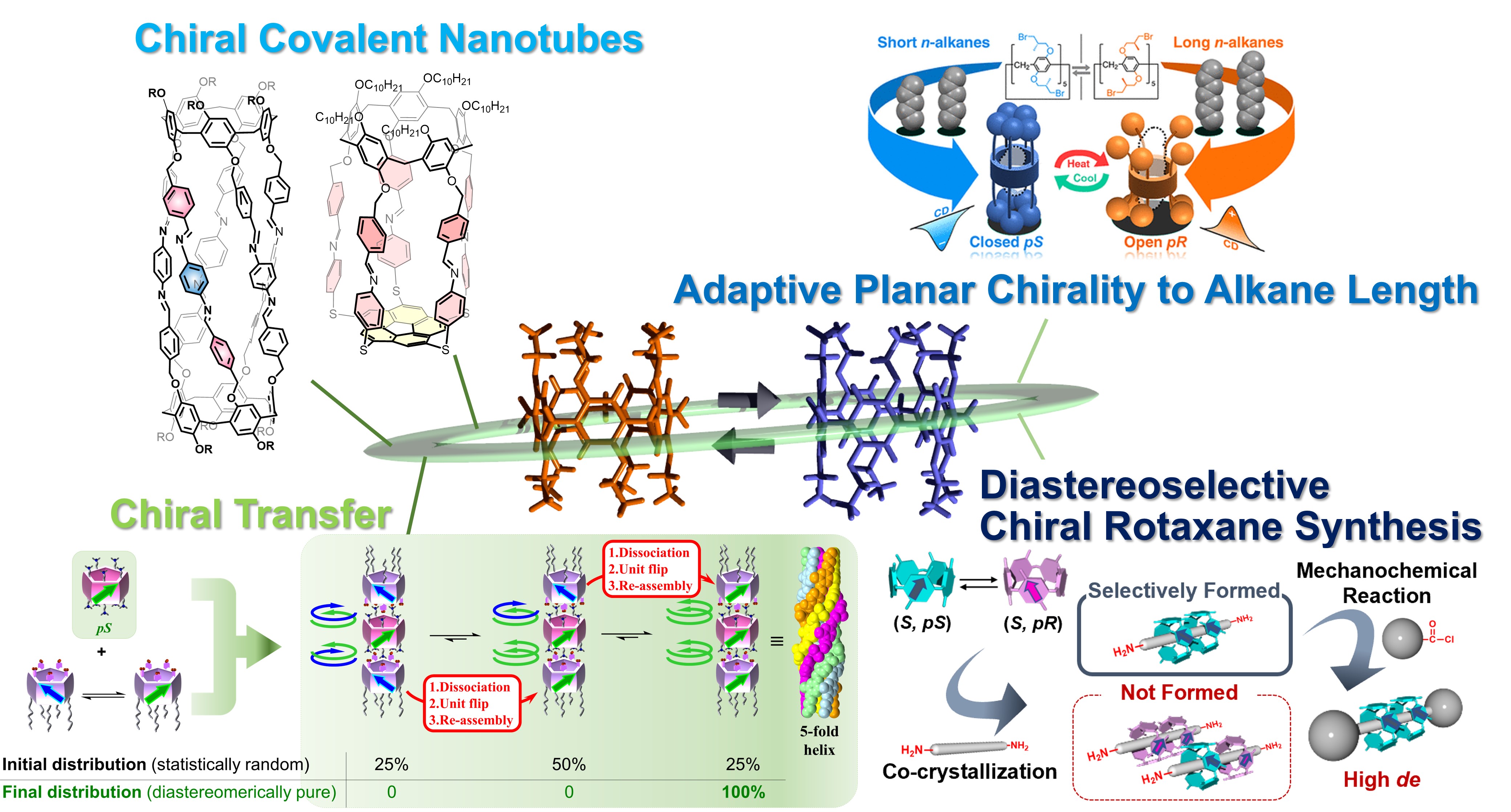
Planar Chirality
Pillar[n]arenes have planar chirality depending on the position of the alkoxy group on the rims. In most cases, racemization proceeds by the rotation of the benzene unit. However, by introducing bulky groups on the rims, rotation of the benzene ring is suppressed, and the pS / pR enantiomer can be isolated by chiral column chromatography. Recently, planar-chiral nanotubes were obtained by co-assembly of planar-chiral deca-amine pillar[5]arene with rim-differentiated racemic penta-acid pillar[5]arene via planar-chiral transfer. Diastereoselective chiral rotaxanes were successfully prepared by co-crystallization of stereodynamic planar-chiral pillar[5]arene with stereogenic carbons at both rims and axles with suitable end groups and lengths and end-capping reactions of the crystalline [3]pseudorotaxane in solvent-free conditions. Discrete organic nanotubes and end-capped nanotubes were prepared by coupling two pillar[5]arenes and connecting between pillar[5]arene and corannulene, respectively, through dynamic covalent bonds. We also reported a system with chirality adapted to n-alkane lengths, using a pillar[5]arene-based macrocyclic host, which contains five stereogenic carbons and five terminal bromine atoms on each rim.