Molecular spatial chemistry based on pillar[n]arenes
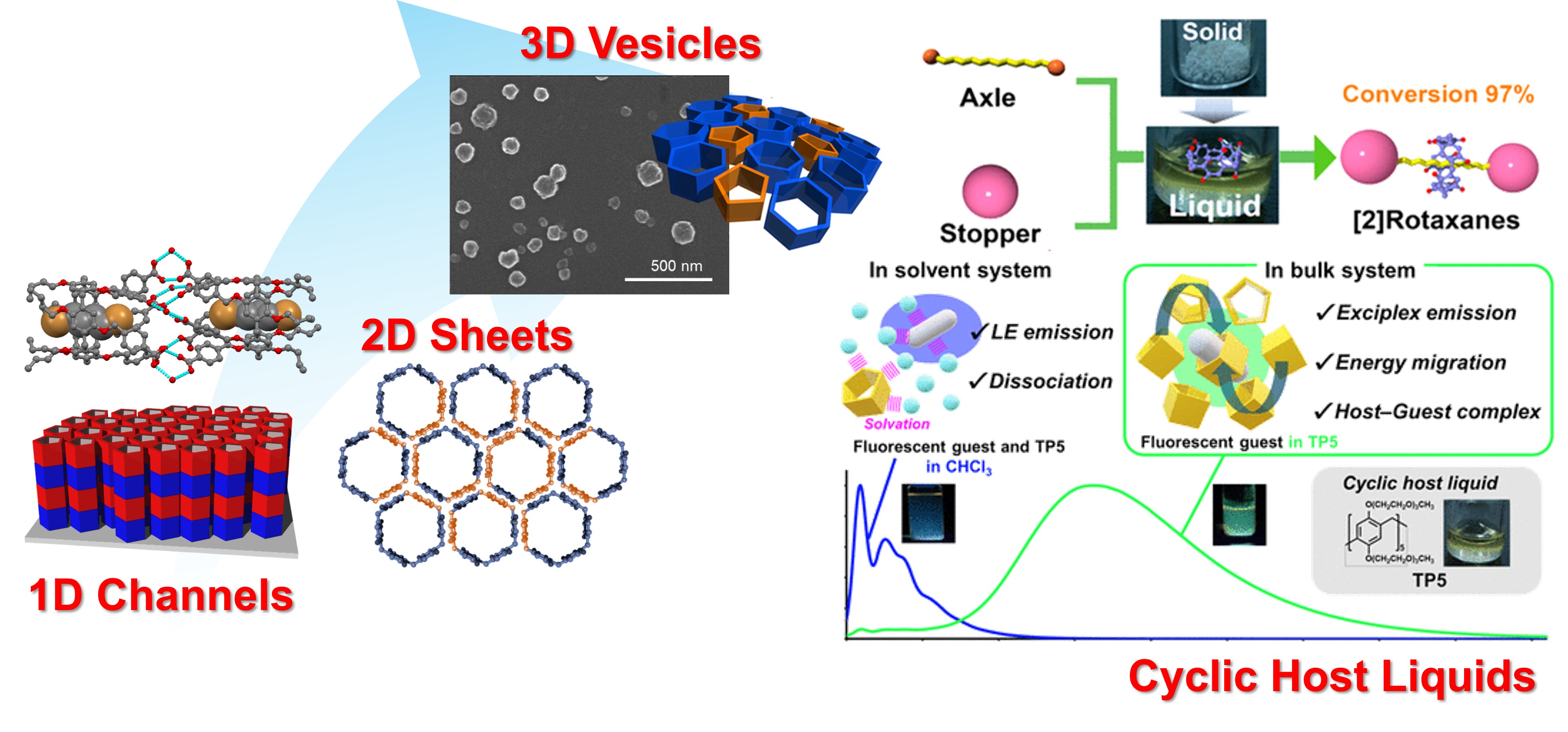
Creation of Molecular Spaces
Since pillar[n]arenes are tubular structures, pillar[n]arenes are useful building blocks to make one-dimensional tubes. For example, pillar[5]arene with five benzoic acids on one side formed a tubular dimer by intermolecular hydrogen bonds between benzoic acids at multiple points. Furthermore, using the layer-by-layer method, we synthesized nanotubes with controlled tube length on the surface. Since pillar[6]arenes have hexagonal structures, they can be assembled to obtain hexagonal two-dimensional sheets. By incorporating pentagonal pillar[5]arenes into the two-dimensional sheet from pillar[6]arenes, we were able to construct a fullerene-like vesicle assembly because pentagons generate curveture. Pillar[5]arenes with triethylene oxide chains are room temperature liquids with a cyclic skeleton because the triethylene oxide chains are liquid. Therefore, when guest molecules are dissolved in the bulk liquid, the guest is efficiently incorporated in the cavity, leading to synthesis of highly efficient interlocking molecules and the unique luminescence behavior due to the proximity of a luminescent acceptor guest to the donor cyclic skeleton.
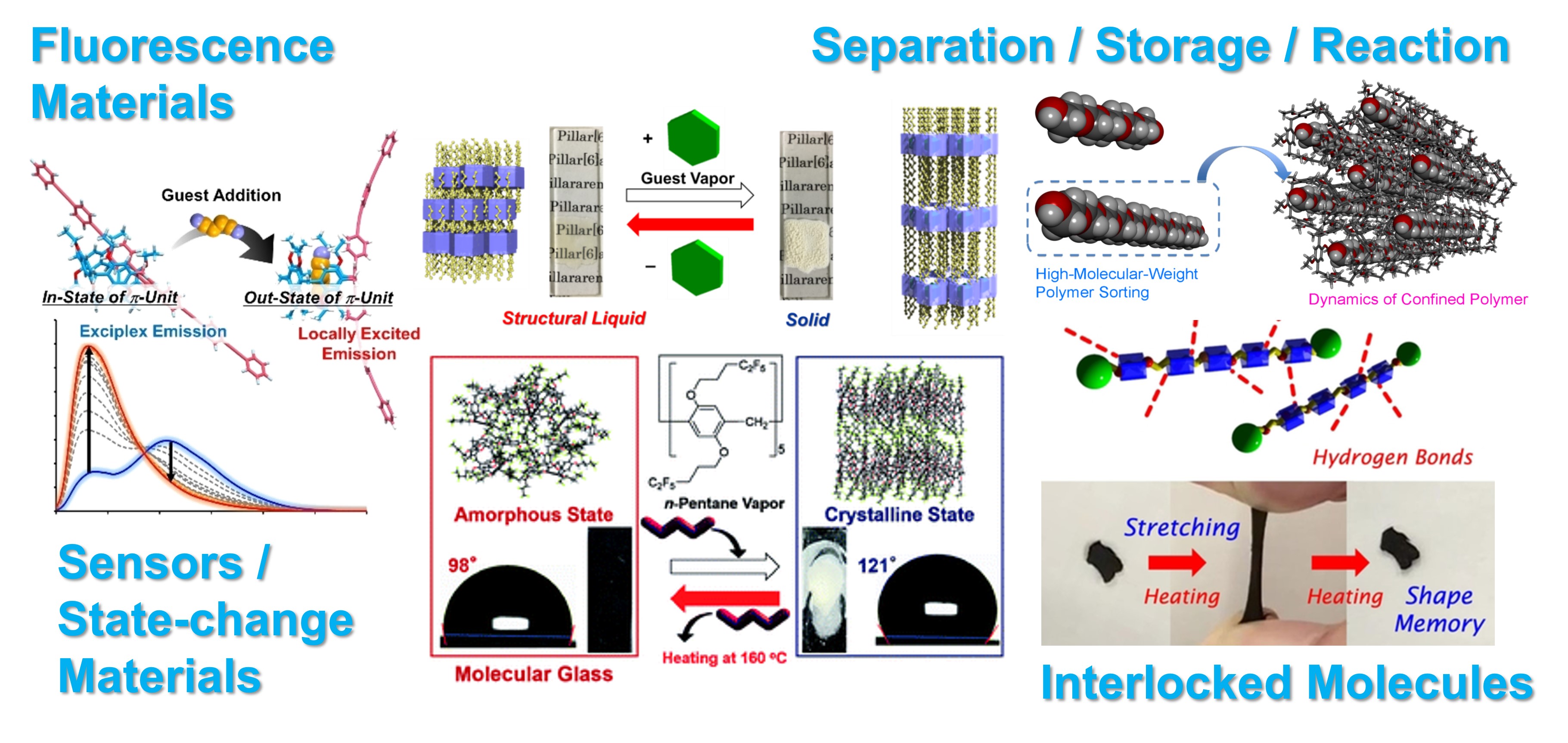
Using Molecular Spaces
Various functional groups can be installed in pillar[n]arenes using the alkoxy groups on the both rims. The functionality enabled to synthesize various functional spatial materials. For example, pillar[n]arenes containing luminescent acceptor molecules showed exciplex emission due to structural relaxation in the excited state, and the exciplex emission was inhibited by the incorporation of guests into the cavity, resulting in localized excitation emission. In addition, when alkyl or fluoroalkyl chains with appropriate lengths are introduced, stimuli-responsive materials that show liquid/solid or amorphous/crystalline structure transitions by incorporation of guest vapor into the cavity can be obtained. Using pillar[n]arene crystals, high molecular weight polymer selective complexation. By threading polymeric chains into the cavities and then blocking the polymer ends to form an interlocking structure, temperature-responsive materials were produced.