Pillar[n]arenes as functional macrocycles with π-electrons
Chiroptical pillar[n]arenes
Pillar[n]arenes have symmetric cylinder shapes with chirality derived from alkoxy substituents at 2,5-positions of benzene ring. They are obtained as a single pair of enantiomers in which all the alkoxy groups are aligned in a symmetric manner. This feature is highly valuable because most oligomeric macrocycles need to be separated from a mixture of stereoisomers.
For a common alkoxy pillar[5]arene, we gave 1) theoretical assignment of the intense CD band (ACIE 2022) and 2) a CPL spectrum (Aggregate 2024).
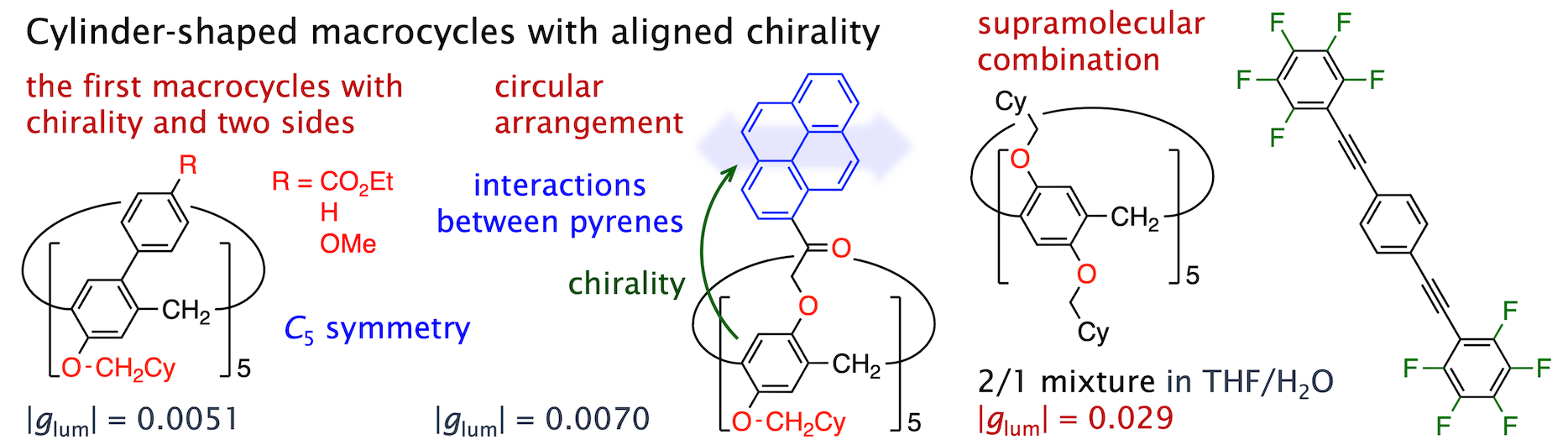
We achieved synthesis of the first discrete macrocycles with chirality and two sides (ACIE 2022).
Other chiroptical molecular systems were developed by circularly arranging luminescent π-planes (CS 2022) and supramolecularly combining with linear π-conjugated molecules (Aggregate 2024).
See also a review on alignment of planar chirality: K. Kato, S. Fa, T. Ogoshi, Angew. Chem. Int. Ed. 2023, 62, e202308316.
Development of unconventional pillar[n]arenes
Pillar[n]arene derivatives mostly have alkoxy substituents because they can be easily synthesized. However, they show 1) poor fluorescence, 2) almost unchanged electronic properties, and 3) weak interplay with introduced functional units apart from the macrocycle cores. To change the situations, pillar[n]arenes need to be modified directly on the macrocycle cores.
We accomplished direct and full substitution of alkoxy groups with aryl substituents (JACS 2023; CS 2024). The “per-arylated” molecules revised our understanding about conformational behaviors and endowed pillar[n]arenes with highly fluorescence properties.
An ESIPT (excited-state intramolecular proton transfer) unit was incorporated into a pillar[n]arenes, providing dual emission responsive to n-hexane vapor in the solid states (BCSJ 2024).
