Catalytic Element-Boryl Addition Reactions
There is an increasing demand for the organoboron compounds in organic synthesis, since a variety of useful transformation reactions of organoboronic acid derivatives have been established. We have focused our attention to the synthesis of functionalized organoboronic acid derivatives by new catalytic reactions, in which the boryl group and the functional group are introduced concomitantly in one step.
Particular efforts have been devoted to silaboration and carboboration, in which a boryl group and additional non-hydrogen groups are introduced simultaneously to unsaturated organic molecules.
2.1. Catalytic Carboboration Reactions †
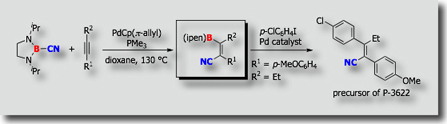
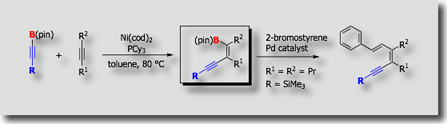
No catalytic carboboration had been reported at the time in spite of the expectation that such reactions are highly useful for the synthesis of organoboron compounds. As the first catalytic carboboration reaction, we established palladium-catalyzed addition of cyanoboranes to carbon-carbon triple bonds (J. Am. Chem. Soc. 2003, Angew. Chem., Int. Ed. 2005). We then found alkynylboration, in which C–B bonds of alkynylboranes add to carbon-carbon triple bonds in a cis fashion (J. Am. Chem. Soc. 2006). These carboboration reactions involve activation of the B–C bond in cyanoboranes and alkynylboranes followed by insertion of alkynes into the transition metal–boron bonds.
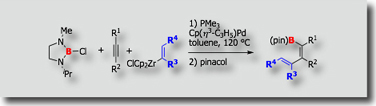
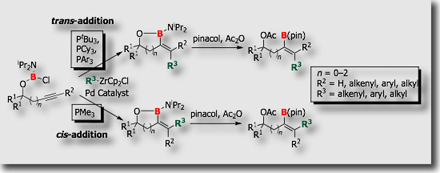
We also established carboboration in which chloroborane and organozirconium reagents were used as a source of the boryl and organic groups, respectively (Chem. Commun. 2008). In this three-component system, activation of the B–Cl bond, which has never been utilized in catalytic reactions, is crucially involved.
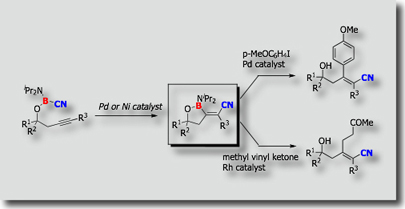
In relation to the three-component carboboration system, a two-component system in which the chloroborane moiety and the triple bonds are tethered by a B–O linkage was reported (J. Am. Chem. Soc. 2008). Interesting reversal of the addition mode was observed: when PMe3 was used as a ligand, cis-addition products were obtained exclusively, while trans-addition products were formed exclusively when PCy3, P(t-Bu)3, or PPh3 was utilized as a ligand. This tethered system has been extended to intramolecular carboboration of C=C bonds more recently (J. Am. Chem. Soc. 2011).
2.2. Catalytic Silaboration Reactions †
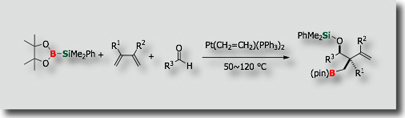
Although no catalytic reaction involving the activation of Si–B bond of silylboranes had been reported before our study (Chem. Commun. 1996), we established that the Si-B bond shows remarkable selectivity and reactivity in the presence of transition metal catalysts (Account: Bull. Chem. Soc. Jpn. 2009).
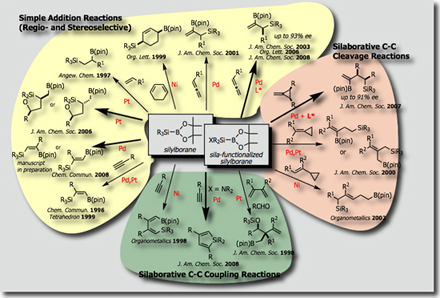
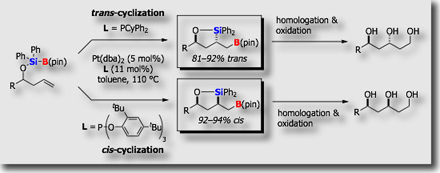
We could establish a variety of catalytic reactions involving (1) simple addition reactions (silaborations), (2) silaborative C–C coupling reactions, and (3) silaborative cleavage of C-C bonds in small rings. In addition, recent success in synthesizing silyl-functionalized silylboranes allowed us to find that (dialkylamino)diorganosilylboranes serves as a synthetic equivalent of “silylene” (J. Am. Chem. Soc. 2009).
We recently found that pyridine derivative underwent dearomatizing silaboration in the presence of a palladium catalyst to give dihydropyridine derivatives under mild reaction conditions (J. Am. Chem. Soc. 2011). This reaction has been extended to our finding on the Rh-catalyzed dearomatizing hydroboration of pyridine derivatives (J. Am. Chem. Soc. 2012).
Typical catalytic reactions with silylborane reagents we have so far developed are shown below.
- Silaboration
- Silaborative Coupling Reaction
- Intramolecular Silaboration
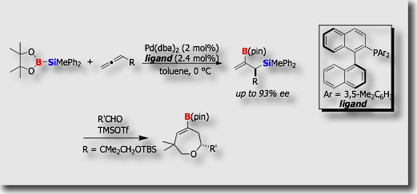
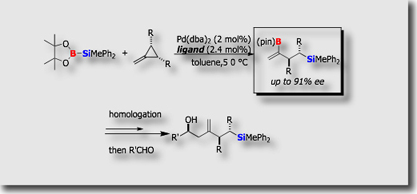
- Catalytic Asymmetric Silaboration
References †
- Ref. 1
- Palladium- and Nickel-Catalyzed Intramolecular Cyanoboration of Alkynes M. Suginome, A. Yamamoto, M. Murakami, J. Am. Chem. Soc. 2003, 125, 6358-6359, 10.1021/ja0349195
- Palladium-Catalyzed Addition of Cyanoboranes to Alkynes Leading to Regio- and Stereoselective Synthesis of β-Boryl-α,β-unsaturated Nitriles M. Suginome, A. Yamamoto, M. Murakami, Angew. Chem., Int. Ed. 2005, 44, 2380-2382, 10.1002/anie.200462961
- Intramolecular Cyanoboration of Alkynes via Activation of Boron-Cyanide Bonds by Transition Metal Catalysts M. Suginome, A. Yamamoto, M. Murakami, J. Organomet. Chem., 2005, 690, 5300–5308, 10.1016/j.jorganchem.2005.05.005
- Reactions of Cyanoboranes with a Palladium–PMeSUB{3}; Complex: Mechanism for the Catalytic Cyanoboration of Alkynes M. Suginome, A. Yamamoto, T. Sasaki, and M. Murakami, Organometallics, 2006, 25, 2911–2913, 10.1021/om060246y
- Synthetic Application of Intramolecular Cyanoboration on the Basis of Removal and Conversion of a Tethering Group by Palladium-Catalyzed Retro-allylation T. Ohmura, T. Awano, M. Suginome, H. Yorimitsu, K. Oshima, Synlett 2008, 423-427, 10.1055/s-2008-1032075
- Ref. 2
- Nickel-Catalyzed Addition of Alkynylboranes to Alkynes M. Suginome, M. Shirakura, A. Yamamoto, J. Am. Chem. Soc. 2006, 128, 14438-14439, 10.1021/ja064970j
- Ref. 3
- Palladium-Catalyzed Carboboration of Alkynes Using Chloroborane and Organozirconium Reagents M. Daini, M. Suginome, Chem. Commun. 2008, 5224-5226, 10.1039/b809433k
- Ref. 4
- Nickel-Catalyzed trans-Alkynylboration of Alkynes via Activation of a Boron-Chlorine Bond A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2005, 127, 15706-15707, 10.1021/ja055396z
- Palladium-Catalyzed trans- and cis-Carboboration of Alkynes Tethered to Chloroborane with Organozirconium Reagents: Ligand-Dependent Complementary Stereoselectivity M. Daini, A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2008, 130, 2918-2919, 10.1021/ja711160h