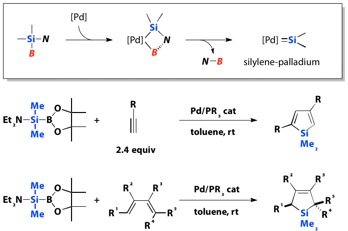
5.1. Catalytic Generation of "Silylene" †
Clean generation of silylene-palladium species is possible by use of silylboranes bearing a dialkylamino group on the silicon atom. The reaction mechanism may involve oxidative addition of the Si-B bond to palladium(0) followed by b-elimination of the amino groups on the silicon atom with boryl group on the palladium atom. The "silylene-palaldium species" give 2,4-disubsituted siloles regioselectively in the reaction with terminal alkynes. The reaction of aminosilylborane with 1,3-dienes affords [4+1] cyclization products stereospecifically with respect to the stereochemistry of the starting 1,3-dienes. The stereospecificity is characteristic to the present reaction system in comparison to known reactions of "free silylene" species generated thermally or photochemically.
5.2. Bis-Silylation †
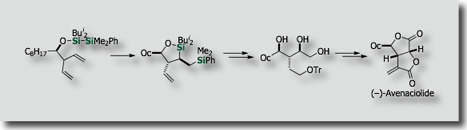
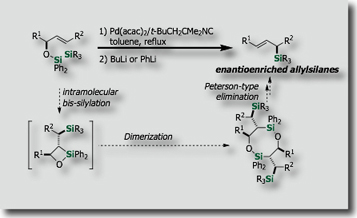
Since silicon is quite abundant in the earth's crust, its utilization in organic synthesis, material science, and medicinal science is highly desirable. We have developed new catalytic silylation reactions on the basis of activation of the silicon-containing σ-bond by transition metal catalysts. For instance, intramolecular bis-silylations have been developed on the basis of our finding on new palladium catalysts, i.e., palladium-isocyanide catalysts [Ref .1], for the activation of the Si-Si bond. The intramolecular reactions enabled the synthesis of enantioenriched allylsilanes [Ref. 2], allenylsilanes [Ref. 3] and polyols [Ref. 4] in stereoselective fashions.
References †
- Ref. 1
- Palladium-Catalyzed Insertion of Isocyanides into the Silicon-Silicon Linkages of Oligosilanes Y. Ito, M. Suginome, T. Matsuura, M. Murakami, J. Am. Chem. Soc. 1991, 113, 8899-8908, 10.1021/ja00023a043
- Palladium(II) Acetate–tert-Alkyl Isocyanide as a Highly Efficient Catalyst for the Inter- and Intramolecular Bis-silylation of Carbon-Carbon Triple Bonds Y. Ito, M. Suginome, M. Murakami, J. Org. Chem. 1991, 56, 1948-1951, 10.1021/jo00005a055
- Intramolecular Bis-silylation of Carbon-Carbon Double Bonds Leading to Stereoselective Synthesis of 1,2,4-Triols M. Murakami, P. G. Andersson, M. Suginome, Y. Ito, J. Am. Chem. Soc. 1991, 113, 3987-3988, 10.1021/ja00010a049
- Stereoselective Intramolecular Bis-Silylation of Alkenes Promoted by Palladium-Isocyanide Catalyst Leading to Polyol Synthesis M. Murakami, M. Suginome, K. Fujimoto, H. Nakamura, P. G. Andersson, Y. Ito, J. Am. Chem. Soc. 1993, 115, 6487-6498, 10.1021/ja00068a003
- Synthesis and Structure of a Nontwisted Tetrakis(organosilyl)ethenes M. Murakami, M. Suginome, K. Fujimoto, Y. Ito, Angew. Chem., Int. Ed. Engl. 1993, 32, 1473-1475, 10.1002/anie.199314731, Angew. Chem. 1993, 105, 1542-1544, 10.1002/ange.19931051041
- Novel Activation of Two Si–Si σ-Bonds in a Molecule by tert-Alkyl Isocyanide–Palladium Complexes M. Suginome, H. Oike, Y. Ito, Organometallics 1994, 13, 4148-4150, 10.1021/om00023a009
- Macrocycles with Regularly Arranged Si–Si Bonds: Ring-Enlargement Oligomerization of Cyclic Disilanes via Palladium-Catalyzed Si-Si σ-Bond Metathesis. M. Suginome, H. Oike, Y. Ito, J. Am. Chem. Soc. 1995, 117, 1665-1666, 10.1021/ja00110a034
- Reactions of Si–Si σ-Bonds with Bis(t-alkyl isocyanide)palladium(0) Complexes. Synthesis and Reactions of Cyclic Bis(organosilyl)palladium Complexes [Headline Article] M. Suginome, H. Oike, S.-S. Park, Y. Ito, Bull. Chem. Soc. Jpn. 1996, 69, 289–299, 10.1246/bcsj.69.289
- Reactions of a Spiro Trisilane with Palladium Complexes: Synthesis and Structure of Tris(organosilyl))Cp Pd(IV) and Bis(organosilyl)(μ-organosilylene)Pd2(II) Complexes. M. Suginome, Y. Kato, N. Takeda, H. Oike, Y. Ito, Organometallics 1998, 17, 495-497, 10.1021/om9710778
- Ref. 2
- New Synthesis of (E)-Allylsilanes with High Enantiopurity via Diastereoselective Intramolecular Bis-Silylation of Chiral Allylic Alcohols M. Suginome, A. Matsumoto, Y. Ito, J. Am. Chem. Soc. 1996, 118, 3061–3062, 10.1021/ja954251x
- Synthesis of Highly Enantio-enriched Allylsilanes via Palladium-catalyzed Intramolecular Bis-Silylation. Determination of the Enantiomeric Excesses through Regio- and Stereoselective Hydroboration with 9-BBN. M. Suginome, T. Iwanami, A. Matsumoto, Y. Ito, Tetrahedron: Asymmetry, 1997, 8, 859-862, 10.1016/S0957-4166(97)00073-6
- Stereoselective Cyclization of Highly Enantio-Enriched Allylsilanes with Aldehydes via Acetal Formation: New Asymmetric Access to Tetrahydropyrans and Piperidines M. Suginome, T. Iwanami, Y. Ito, J. Org. Chem. 1998, 63, 6096-6097, 10.1021/jo981173y
- Asymmetric Synthesis of 2,3-Disubstituted Oxepanes via Acetalization-Cyclization of an Enantioenriched Functionalized Allylsilane with Aldehydes M. Suginome, T. Iwanami, Y. Ito, Chem. Commun. 1999, 2537-2538, 10.1039/a908603j
- Asymmetric Synthesis of Cyclic Alkenes via Cyclization of Enantioenriched Allylsilanes M. Suginome, T. Iwanami, A. Yamamoto, and Y. Ito, Synlett, 2001, 1042-1045, 10.1055/s-2001-14656
- Solid-Phase Synthesis and Asymmetric Reactions of Polymer-Supported Highly Enantioenriched Allylsilanes M. Suginome, T. Iwanami, and Y. Ito, J. Am. Chem. Soc. 2001, 123, 4356-4357, 10.1021/ja005865r
- Stereoselective Synthesis of Highly Enantioenriched (E)-Allylsilanes by Palladium-Catalyzed Intramolecular Bis-Silylation: 1,3-Chirality Transfer and Enantienrichment via Dimer Formation M. Suginome, T. Iwanami, Y. Ohmori, A. Matsumoto, Y. Ito, Chem. Eur. J., 2005, 11, 2954-2965, 10.1002/chem.200401031
- Ref. 3
- Palladium-Catalyzed Intramolecular Bis–Silylation of Propargylic Alcohols: A New Stereospecific Access to Chiral Allenylsilanes M. Suginome, A. Matsumoto, Y. Ito, J. Org. Chem. 1996, 61, 4884-4885, 10.1021/jo960778w
- Ref. 4
- Diastereoselective Intramolecular Bis-Silylation of a Carbon-Carbon Double Bond. A Highly Stereocontrolled Synthesis of (-)-Avenaciolide M. Suginome, Y. Yamamoto, K. Fujii, Y. Ito, J. Am. Chem. Soc. 1995, 117, 9608–9609, 10.1021/10.1021/ja00142a047
- Intramolecular Bis-Silylation of Alkenes Catalyzed by Palladium(0) tert-alkyl isocyanide Complex. Stereoselective Synthesis of Polyols Y. Ito, M. Suginome, Pure Appl. Chem. 1996, 68, 505-508, 10.1351/pac199668030505
5.3. New Iminium Ion generators for Amination Reactions †
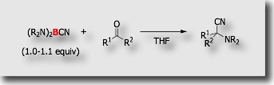
In the course of our study on the synthetic application of cyanoboranes, we found interesting reactivity of bis(dialkylamino)cyanoboranes toward carbonyl compounds. In 1:1 reaction of the cyanoboranes with aldehydes at room temperature, α-dialkylamino nitriles were obtained in high yields [Ref. 1].
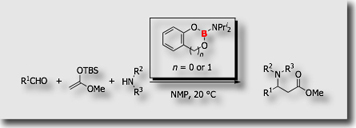
We designed aminoboranes bearing bulky diisopropyl groups on the boron atom. The aminoboranes served as a universal iminium ion generator, allowing use of free sec-amines in the Mannich-type reaction as the source of the amino group through facile amino group exchange on the boron atom [Ref. 2].
The reaction protocol could be extended to other amination reactions, such as reductive amination [Ref. 3] and Ugi reactions [Ref. 4].
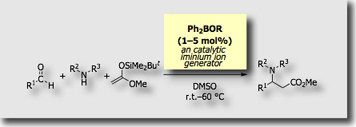
More recently, we found that diphenylborinic acid derivatives serve as “catalytic” iminium ion generators in the Mannich-type reactions [Ref. 5].
References †
- Ref. 1
- Bis(dialkylamino)cyanoboranes: highly efficient reagents for the Strecker-type aminative cyanation of aldehydes and ketones. M. Suginome, A. Yamamoto, Y. Ito, Chem. Comm. 2002, 1392-1393, 10.1039/b203645b
- New Look at Boron Enolate Chemistry: Aminative C–C Bond Formation Using Diaminoboron Enolate with Aldehyde M. Suginome, L. Uhelin, A. Yamamoto, M. Murakami, Org. Lett. 2004, 6, 1167-1169, 10.1021/ol0497436
- Ref. 2
- Aminoboranes as "Compatible" Iminium Ion Generators in Aminative C-C Bond Formations M. Suginome, L. Uhelin, M. Murakami, J. Am. Chem. Soc. 2004, 126, 13196-13197, 10.1021/ja045827y
- Aminoboranes as New Iminium Ion Generators in Amination Reactions M. Suginome, Pure Appl. Chem. 2006, 78, 1377-1387, 10.1351/pac200678071377
- Ref. 3
- Reductive Amination of Aldehydes Using Aminoboranes as Iminium Ion Generators M. Suginome, Y. Tanaka, and T. Hasui, Synlett 2006, 1047–1050, 10.1055/s-2006-939070
- Ref. 4
- Acid-free, Aminoborane-mediated Ugi-type Reaction Leading to General Utilization of Secondary Amines Y. Tanaka, Tomoaki Hasui, M. Suginome, Org. Lett. 2007, 9, 4407-4410, 10.1021/ol701570c
- Ref. 5
- Diarylborinic Acid Derivatives as a Catalytic Iminium Ion Generator in the Mannich-type Reaction Using sec-Amines, Aldehydes, and Ketene Silyl Acetals Y. Tanaka, T. Hasui, M. Suginome, Synlett, 2008, 1239–1242, 10.1055/s-2008-1072724