Cross-Coupling-Based Synthetic Strategy
3.1. "Boron-Masking Strategy" for the Module-Based Iterative Cross-Coupling †
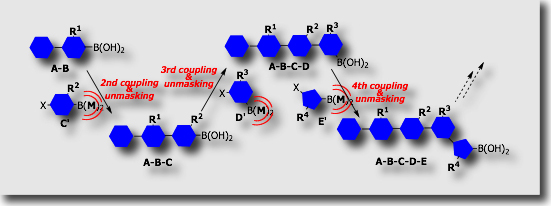
With the increasing demands for the organoboronic acid derivatives in synthetic organic chemistry, much effort is devoted to the development of their synthetic methods as well as their new reactions. Our particular attention has focused on the control of the reactivities of organoboronic acid derivatives by introduction of particular “ligands” on the boron atoms. For example, masking, i.e., temporary protection, of the boronyl groups (B(OH)2) in the Suzuki–Miyaura coupling would lead to an iterative coupling system in which masked modules are repeatedly coupled.
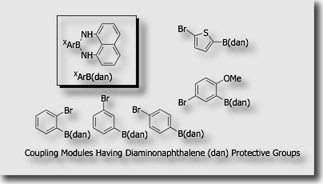
We established the first protective group for organoboronic acids in the Suzuki-Miyaura coupling (J. Am. Chem. Soc. 2007). We utilized 1,8-diaminonaphthalene (DAN) as a protective group of the boronyl groups. The protective group could be easily installed in high yield by the condensation of organoboronic acids with 1,8-diaminonaphthalene and could be deprotected in high yield by treatment with aqueous acids.
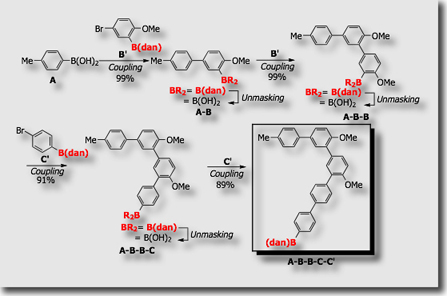
Highly selective, seven-step synthesis of quinquearyl A-B-B-C-C’ was demonstrated by applying the 1,8-diaminonaphthalene-based iterative coupling system, in which coupling modules B’ and C’ are used with arylboronic acids A as a starting unit.
The DAN-protection can be installed into the boron reagents used in the catalytic boron-carbon bond formations. An example is shown by the unsymmetrical diboron (pin)B-B(dan), which undergoes Ir- and Pt-catalyzed regioselective diboration of terminal alkynes (J. Am. Chem. Soc. 2010). By virtue of the regioselective introduction of less reactive B(dan) group into the terminal position, the diboration products undergo selective cross-coupling at the potentially less reactive internal position, where the B(pin) group is located.
3.2. Complementary Stereospecificity in Cross-Coupling of Chiral α-Aminoalkylboronic Acid Derivatives †
Under construction.
References †
- Ref. 1
- Boron-Masking Strategy for the Selective Synthesis of Oligoarenes via Iterative Suzuki-Miyaura Coupling H. Noguchi, K. Hojo, M. Suginome, J. Am. Chem. Soc. 2007, 129, 758-759, 10.1021/ja067975p
- Differentially Protected Benzenediboronic Acids: Divalent Cross-Coupling Modules for the Efficient Synthesis of Boron-Substituted Oligoarenes H. Noguchi, T. Shioda, C.-M. Chou, M. Suginome, Org. Lett. 2008, 10, 377-380, 10.1021/ol702420x
Acknowledgement †
This work was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT). Grant-in-Aid for Scientific Research (A), KAKENHI (19205007). Link