メンバー
清水 大貴 (Daiki Shimizu, Ph.D.)

京都大学大学院工学研究科 合成・生物化学専攻
合成化学講座 物理有機化学分野(松田研究室)
助教 / 京都大学創発PI
工学研究科次世代学際院 次世代研究者
京都大学教育研究活動データベース
〒615-8510
京都市西京区京都大学桂 A4棟325号室
e-mail: dshimizu(at)sbchem.kyoto-u.ac.jp
Tel: 075-383-2746
Fax: 075-383-2741
専門分野:構造有機化学、分子磁性
研究テーマ:新奇な電子構造の設計に基づく機能性π電子系の創製
研究テーマ:新奇な電子構造の設計に基づく機能性π電子系の創製
学年や学内外問わず研究室の見学はいつでも歓迎しています。
まずは気軽にメールでご連絡ください。
まずは気軽にメールでご連絡ください。
学歴
| 2010-03 | 愛知県立刈谷高等学校 卒業 |
|---|---|
| 2014-03 | 京都大学理学部 卒業 |
| 2016-03 | 京都大学大学院理学研究科 化学専攻 修士課程 修了 |
| 2019-03 | 京都大学大学院理学研究科 化学専攻 博士後期課程 修了(大須賀篤弘 教授) |
| 2019-03 | 博士(理学) 学位論文 "Chemistry of Stable Open-shell Porphyrinoids" |
職歴
| 2016-04/2019-03 | 日本学術振興会特別研究員(DC1) |
|---|---|
| 2017-08/2017-11 | 米国Northwestern大学 Visiting Scholar (Michael R. Wasielewski Group) |
| 2019-04/現在 | 京都大学大学院工学研究科 合成・生物化学専攻 助教 |
| 2025-10/現在 | JST 創発的研究支援事業 創発研究者(グンパネル)、京都大学創発PI |
受賞歴
| 2016-10 | 第 7 回大津会議アワードフェロー(No. 107) |
|---|---|
| 2017-03 | 学生講演賞@日本化学会第 97 春季年会 |
| 2017-08 | ポスター賞@第 48 回構造有機若手の会夏の学校 |
| 2018-09 | RSC Advances賞@第 29 回基礎有機化学討論会 |
| 2018-12 | Chemical Reviews Poster Prize@The 10th Singapore |
| 2020-02 | 第 36 回 井上研究奨励賞 |
| 2023-12 | 第 36 回 有機合成化学協会 三菱ケミカル 研究企画賞 「ボトムアップ型分子設計に基づく赤外エレクトロクロミズム材料の設計と自在制御」 |
| 2025-02 | 第 9 回 福井謙一奨励賞 |
原著論文
TOC表示:
あり
|
なし
-
67. X. Wan, Y. Rao, L. Xu, B. Yin, M. Zhou, D. Shimizu, A. Osuka, J. Song
“NiII and CuII Complexes of 5-Oxa-, 5-Sulfa-, 5-Selena-, and 5-Telluracorroles”
Org. Lett. 2025, 27, 10093-10097. [DOI: 10.1021/acs.orglett.5c03160]

-
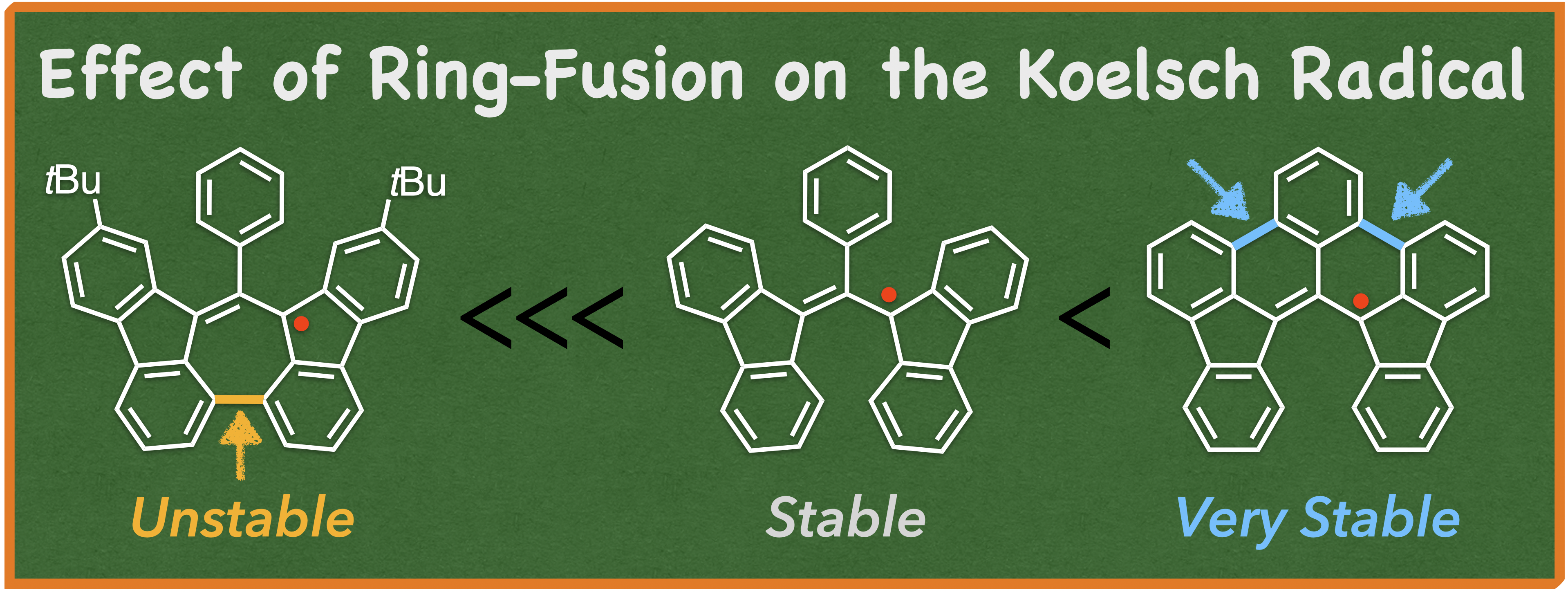
66. D. Shimizu, K. Matsuda
“Modulation of Koelsch Radical Stability and Aromaticity through Nonhexagonal Ring Fusion”
Org. Lett. 2025, 27, 9517-9521. [DOI: 10.1021/acs.orglett.5c03054]
-
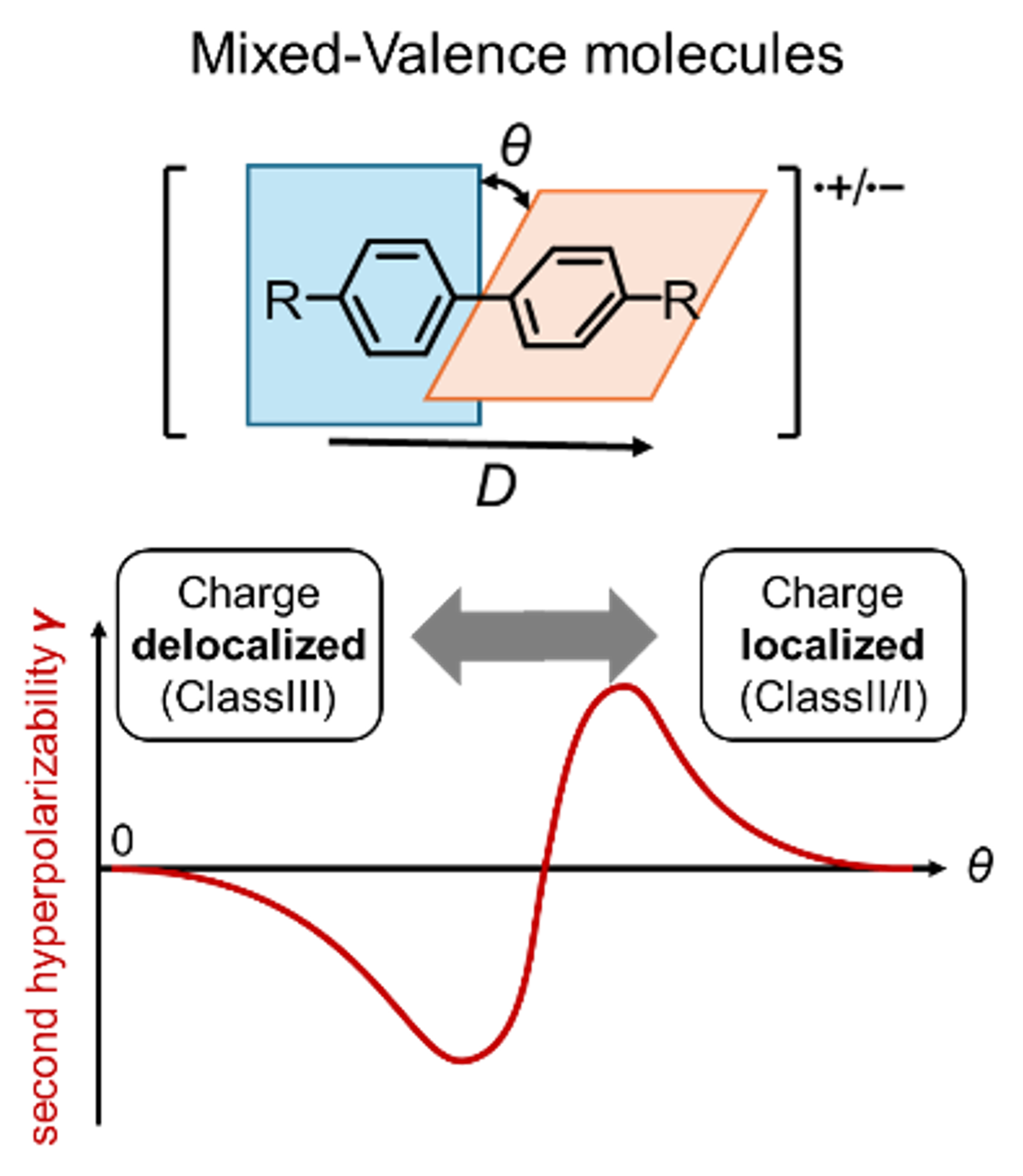
65. T. Shinozuka, D. Shimizu, K. Matsuda
“Computational Study on the Relationship between Charge Localization and Second Hyperpolarizability in Mixed-Valence Systems”
Chem. Eur. J. 2025, 31, e202501019. [DOI: 10.1002/chem.202501019]
-
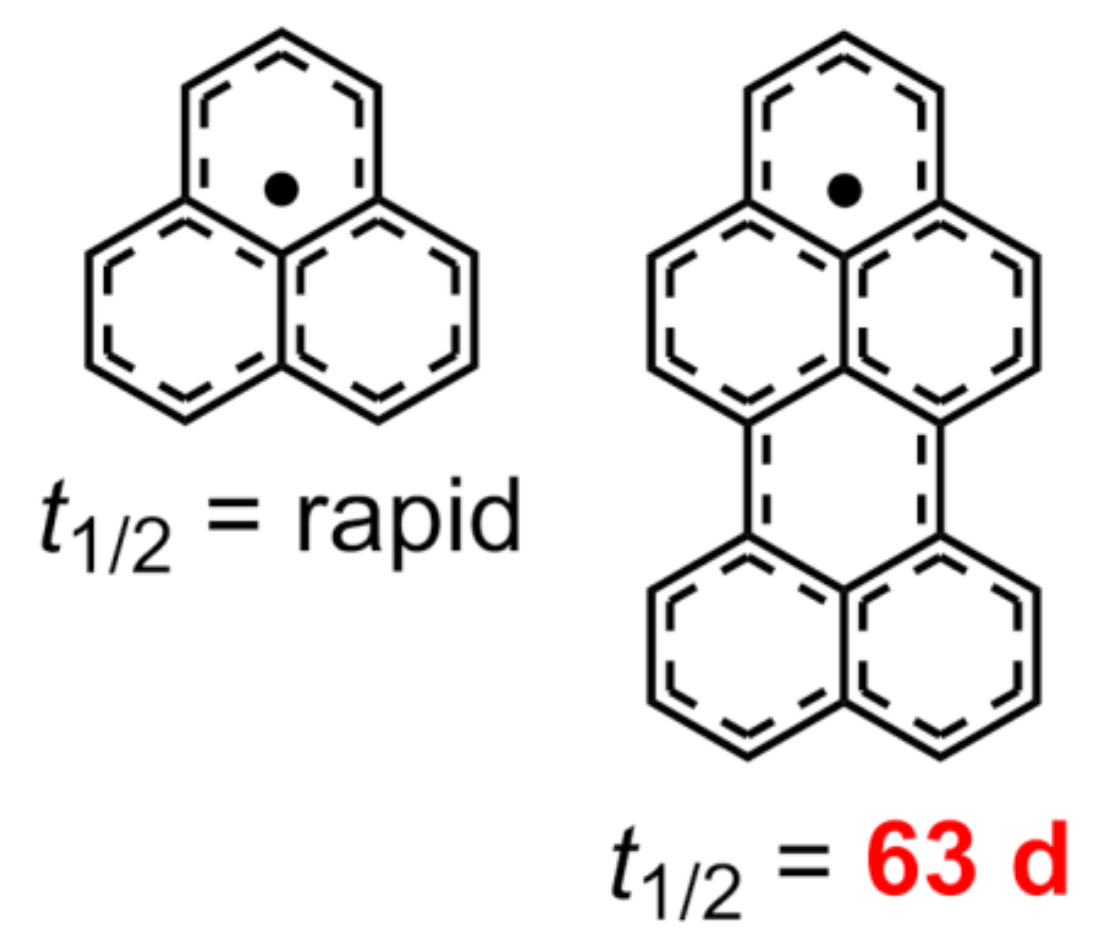
64. Y. Ono, Y. Goto, Y. Tani, K. Nakasuji, K. Sato, T. Takui, D. Shimizu, K. Matsuda, T. Kubo
“Benzo[cd]perylenyl: A π-Expanded Phenalenyl Radical with Enhanced Aggregation Enthalpy and Stability”
Asian J. Org. Chem. 2025, 14, e202500300. [DOI: 10.1002/ajoc.202500300]
-
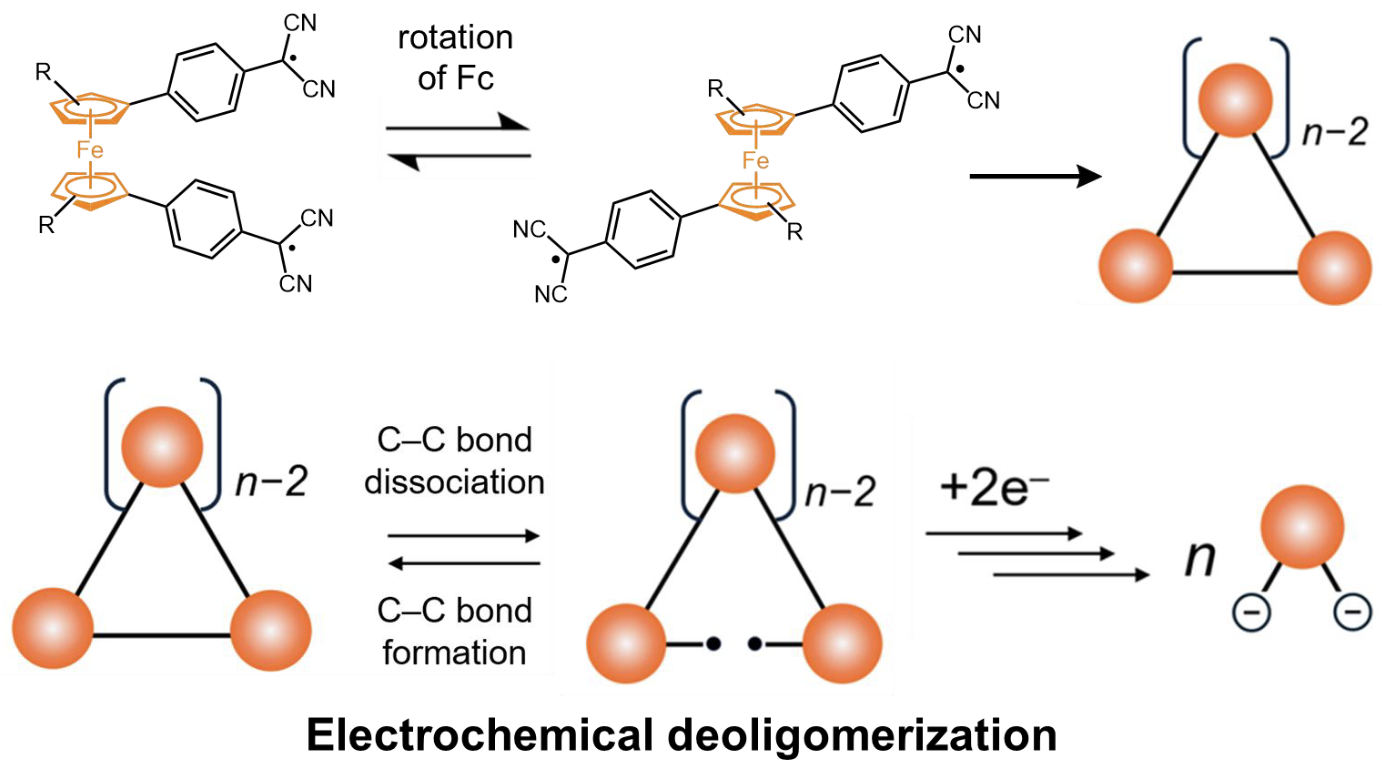
63. D. Sakamaki, K. Asano, D. Shimizu, H. Fujiwara
“Redox Control of Radical-Based Dynamic Covalent Chemistry: Macrocycle Formation and Electrochemical Deoligomerization of Bis(Dicyanomethyl Radical)-Substituted Ferrocene”
Asian J. Org. Chem. 2025, 14, e202500253. [DOI: 10.1002/ajoc.202500253]
-
62. D. Sakamaki, K. Tsubono, M. Nakamura, D. Shimizu, Y. Matsui, H. Ikeda, K. Furukawa, H. Fujiwara
“Intermolecular Toroidal Conjugation in Cylindrically Aligned Sixteen π-Planes Formed by Self-dimerization of Octa-substituted Phthalocyanines”
Angew. Chem. Int. Ed. 2025, 64, e202504353. [DOI: 10.1002/anie.202504353]
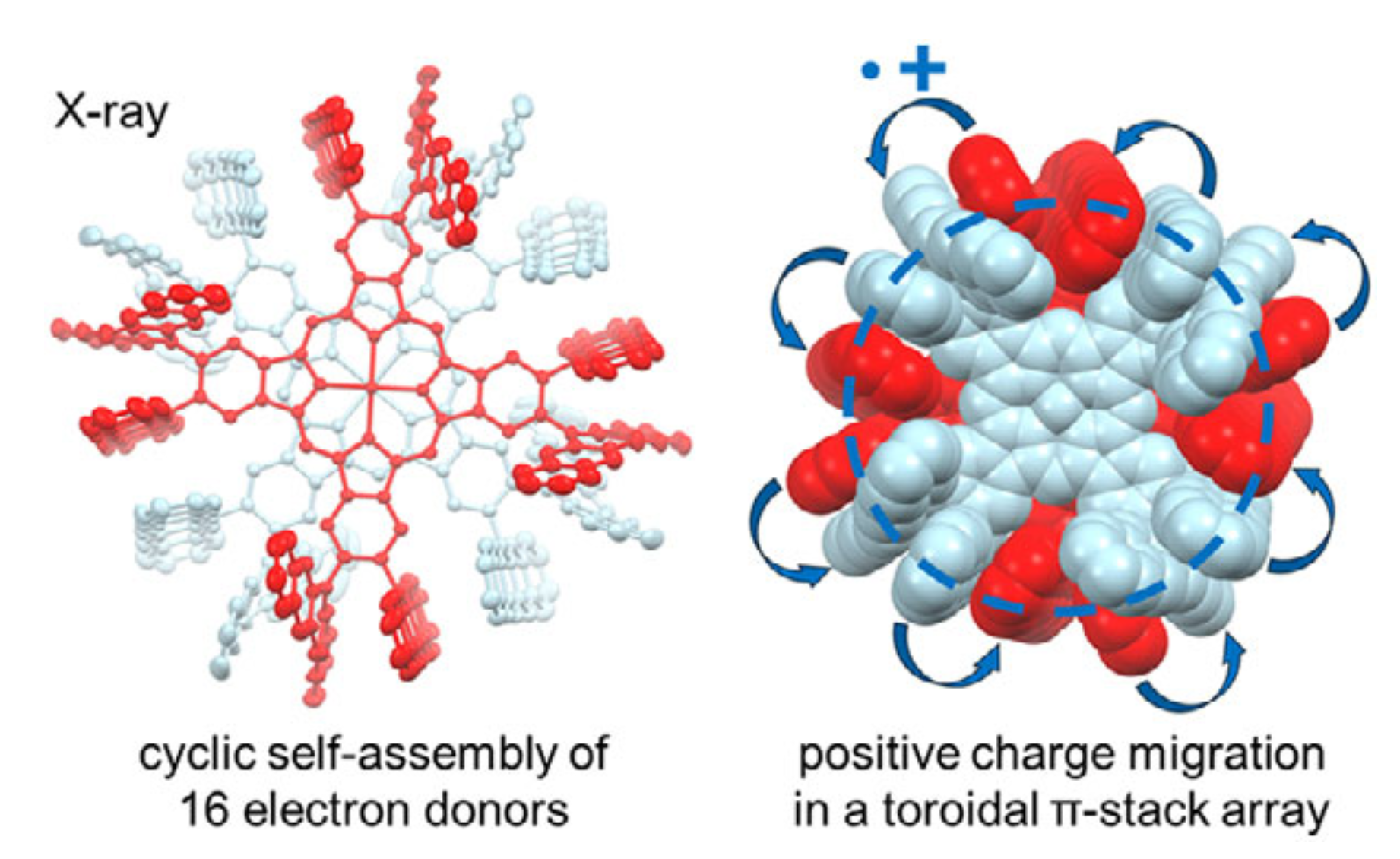
-
61. Y. Fujimoto, D. Shimizu, K. Matsuda
“Rational Design of a Metal-free Organic Radical Cation Exhibiting Mid-IR π-π* Transition based on the Exchange Interaction”
J. Phys. Chem. Lett. 2025, 16, 11242-11247. [DOI: 10.1021/acs.jpclett.5c02562]
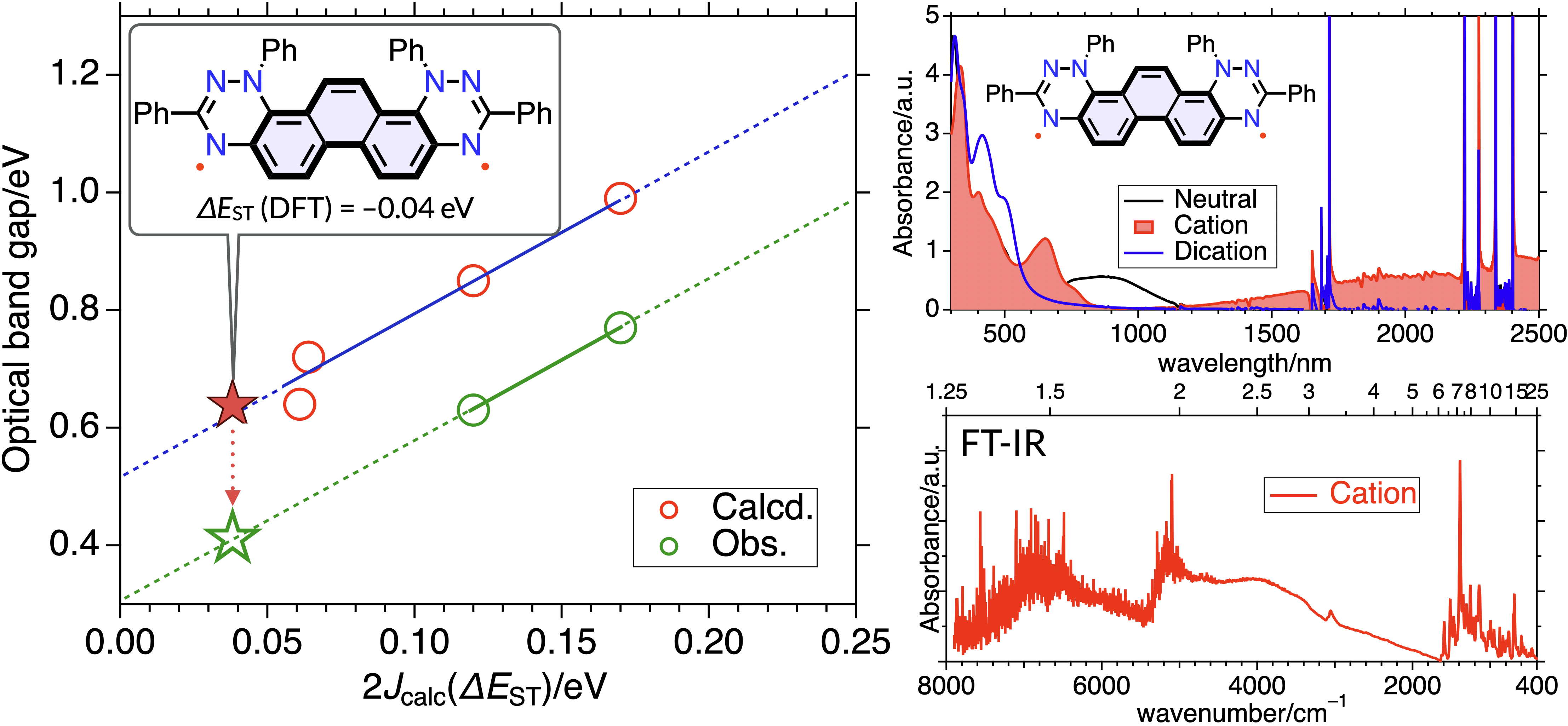
-
60. K. Tajima, C. Bucher, D. Shimizu, N. Fukui, H. Shinokubo
“Captodative Approach to Stable Nitrogen-Centered Radicals, Anions, and Cations Exhibiting Near-Infrared Electrochromism”
JACS Au 2025, 5, 1421-1428. [DOI: 10.1021/jacsau.5c00037]
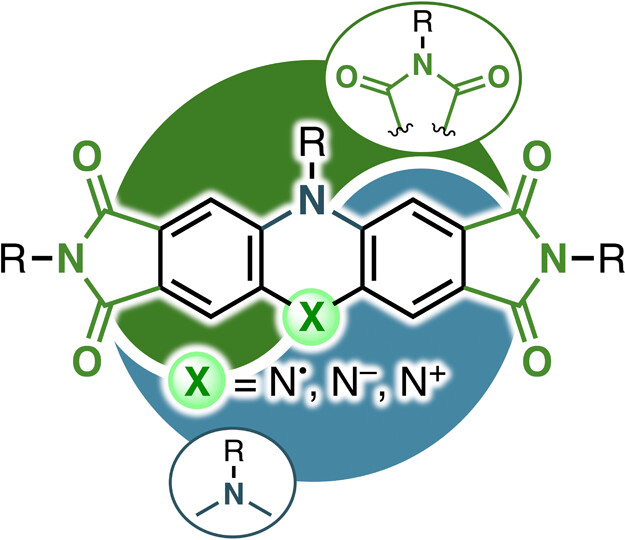
-
59. T. Shinozuka, D. Shimizu, K. Matsuda
“The effect of peri-fusion position on the single molecular conductivity of AGNRs theoretically evaluated by decay constant of exchange interaction”
Chem. Lett. 2024, 53, upae225. [DOI: 10.1093/chemle/upae225]
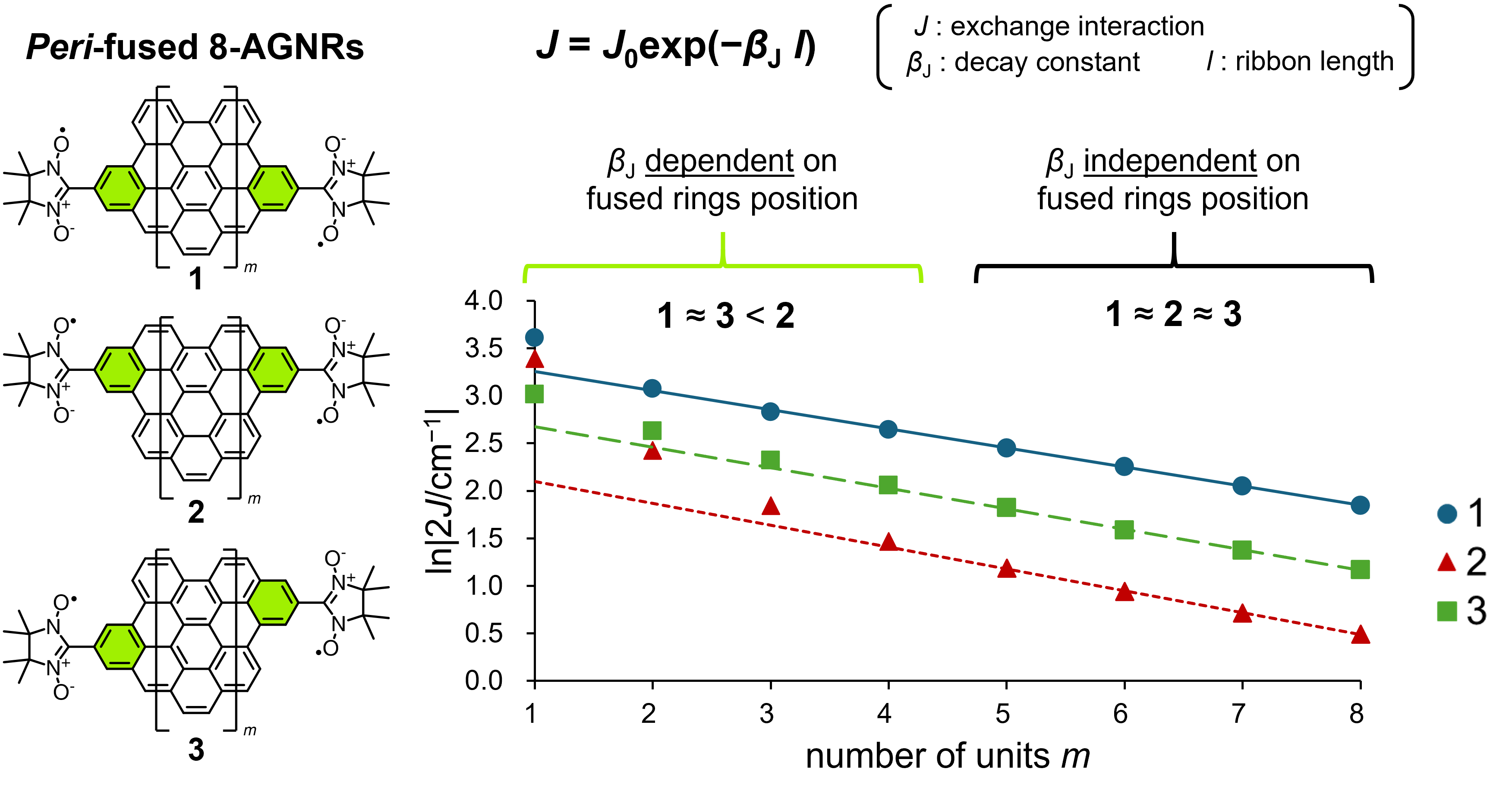
-
58. K. Wang, A. Ghosh, D. Shimizu, H. Takano, M. Ishida, R. Kishi, H. Shinokubo
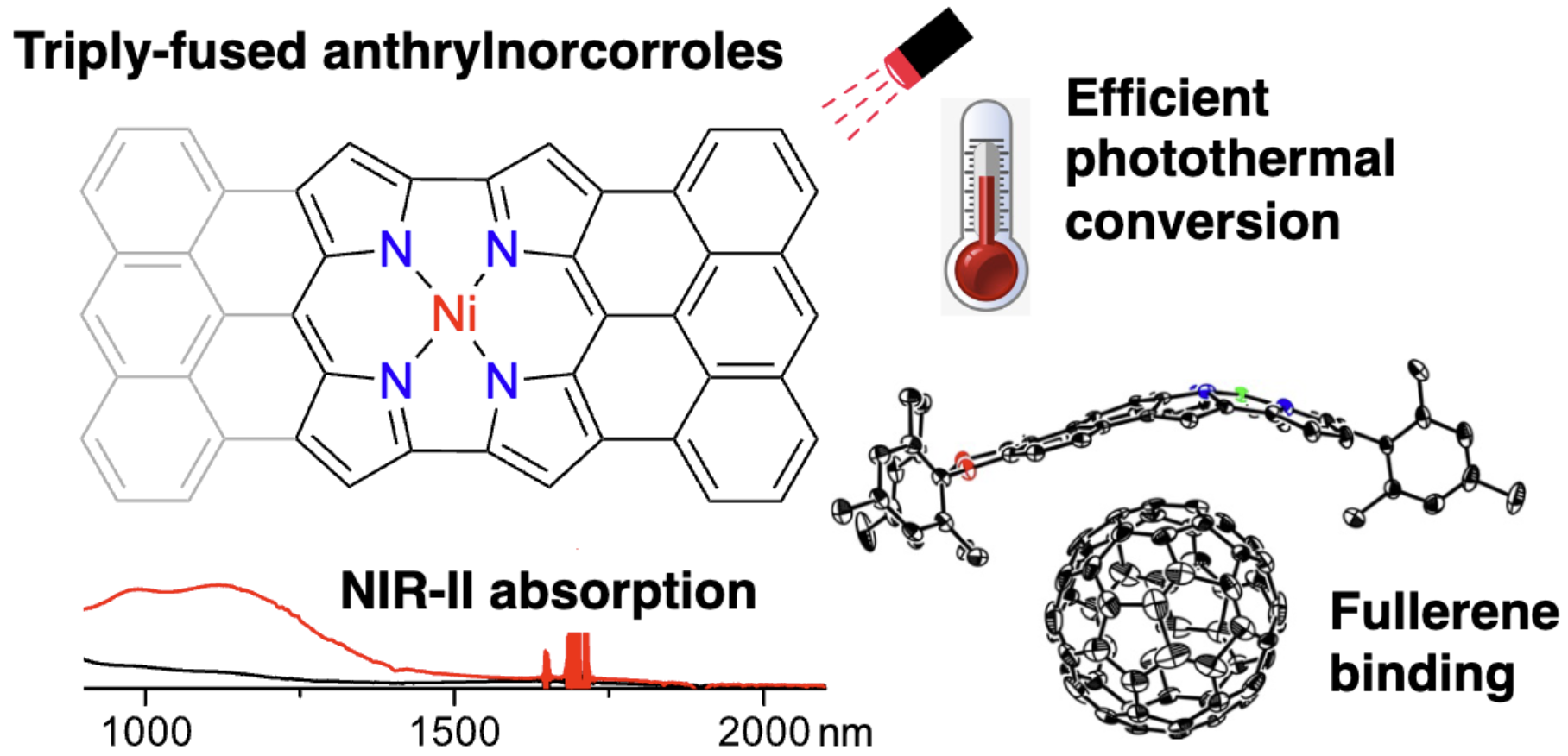
“Bowl-Shaped Anthracene-Fused Antiaromatic Ni(II) Norcorrole: Synthesis, Structure, Assembly with C60, and Photothermal Conversion”
Angew. Chem. Int. Ed. 2025, 64, e202419289. [DOI: 10.1002/anie.202419289] (Open access)
*Press Release
-
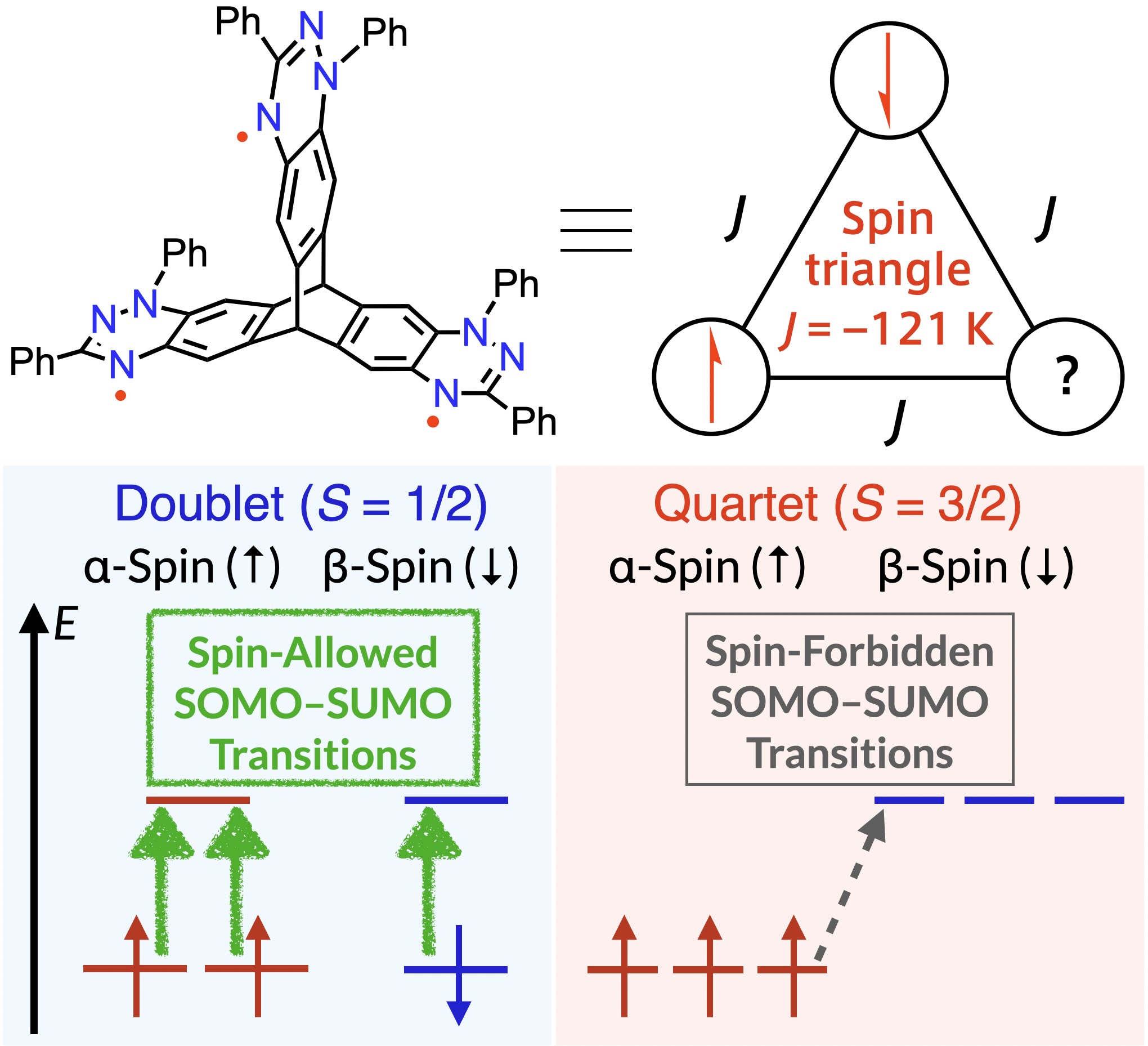
57. T. Aoki, H. Sotome, D. Shimizu, H. Miyasaka, K. Matsuda
“Propeller-Shaped Blatter-Based Triradicals: Distortion-Free Triangular Spin System and Spin-State-Dependent Photophysical Properties”
Angew. Chem. Int. Ed. 2025, 64, e202418655 (Hot Paper). [DOI: 10.1002/anie.202418655]
-
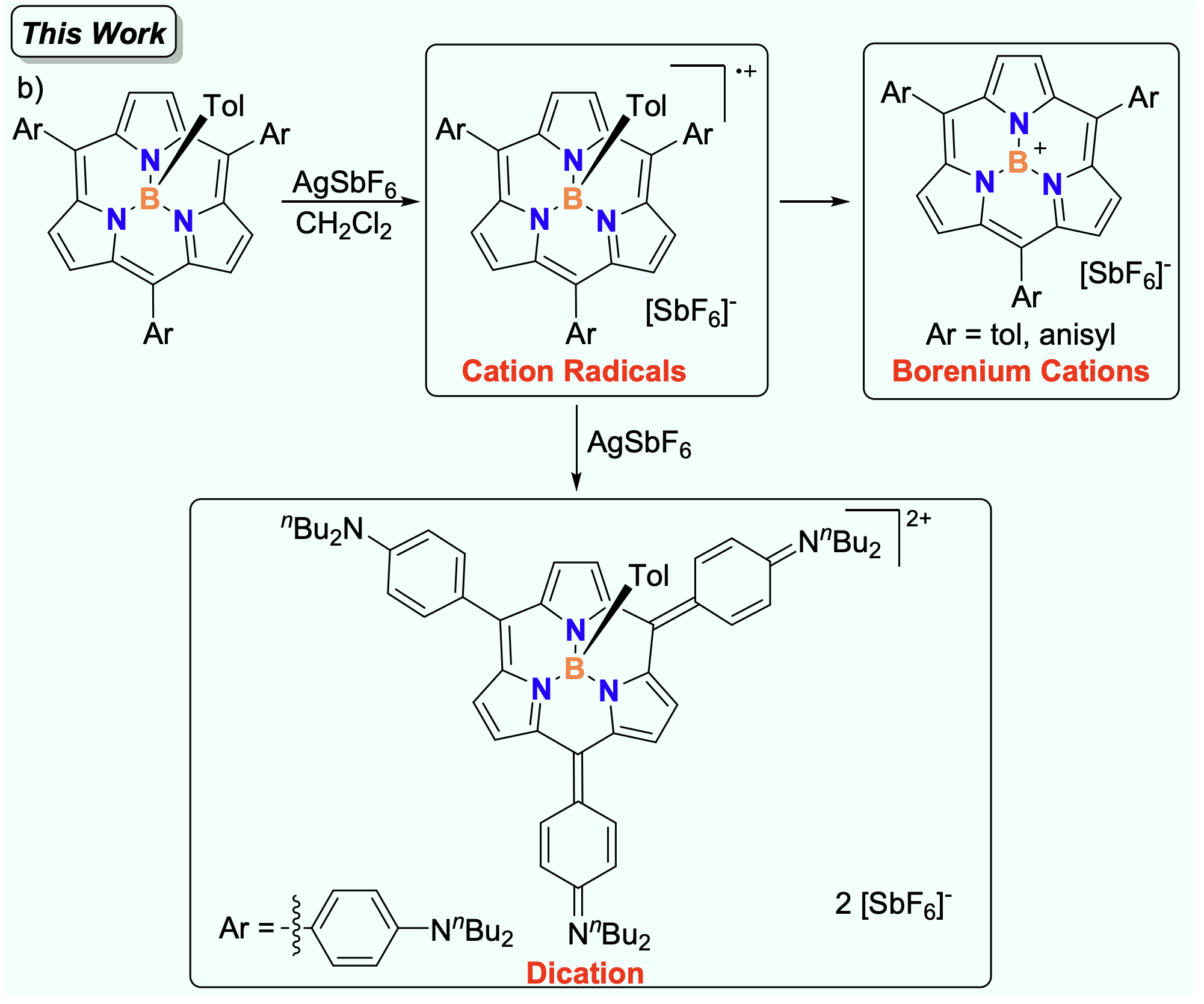
56. Z. Xie, X. Ji, X. Zeng, D. Shimizu, T. Tanaka, Y. Rao, M. Zhou, L. Xu, A. Osuka, J. Song
“Cation Radicals, Borenium Cations, and Dication from Oxidation of B-Tolyl BIII Subporphyrins”
Org. Chem. Front. 2025, 12, 1565-1571. [DOI: 10.1039/D4QO02291B]
-
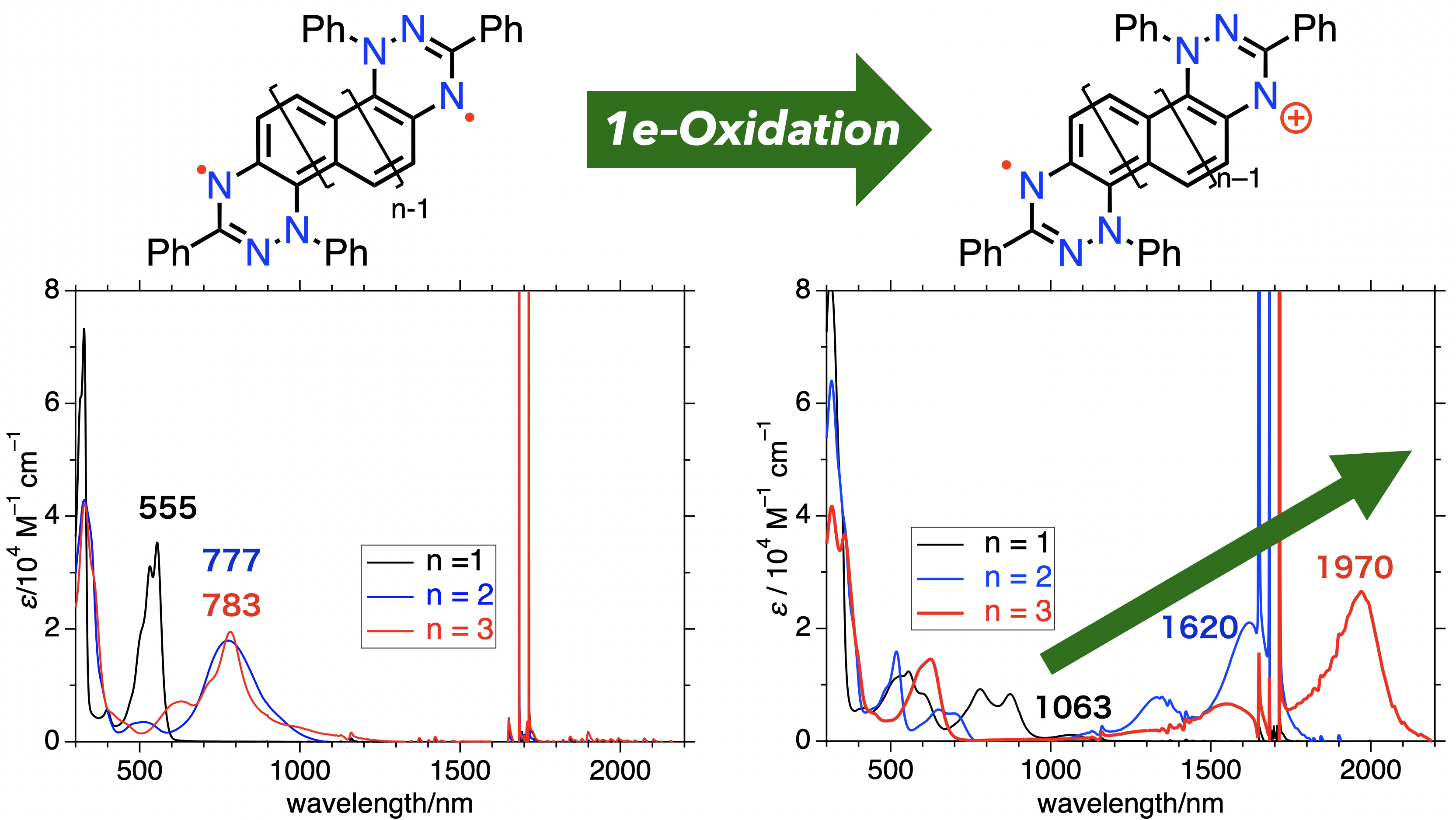
55. T. Yamada. D. Shimizu, K. Matsuda
“Oxidation of Weakly Interacting Diradicals: an Approach for Strong and Tunable NIR-absorbing Dyes based on Small Chromophores”
J. Phys. Chem. Lett. 2024, 15, 9175-9182. [DOI: 10.1021/acs.jpclett.4c02212]
-
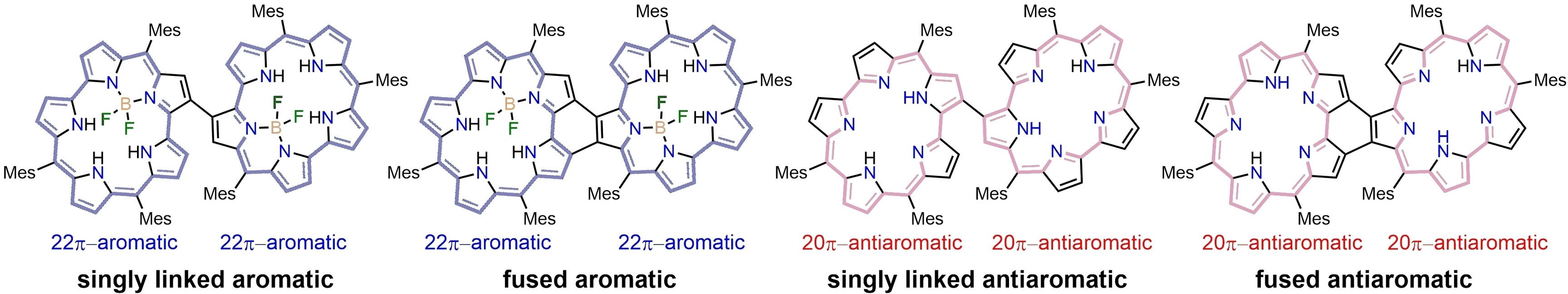
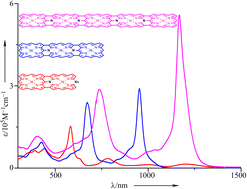
54. Y. Liu, T. Tanaka, D. Shimizu, Y. Rao, L. Xu, B. Yin, M. Zhou, J. Song, A. Osuka
“Fused Aromatic and Antiaromatic Smaragdyrin Dimers”
Angew. Chem. Int. Ed. 2024, 63, e202408478. [DOI: 10.1002/anie.202408478]
-
53. L. Wang, Z. Liao, P. Lin, Y. Jia, L. Liu, L. Xu, M. Zhou, B. Yin, Y. Rao, A. Nakai, T. Tanaka, D. Shimizu, A. Osuka, J. Song.
“Synthesis of NiII Porphyrin—NiII 5,15-Diazaporphyrin Hybrid Tapes“
Chem. Sci. 2024, 15, 10207-10213. [DOI: 10.1039/D4SC01450B] (Open access)
-
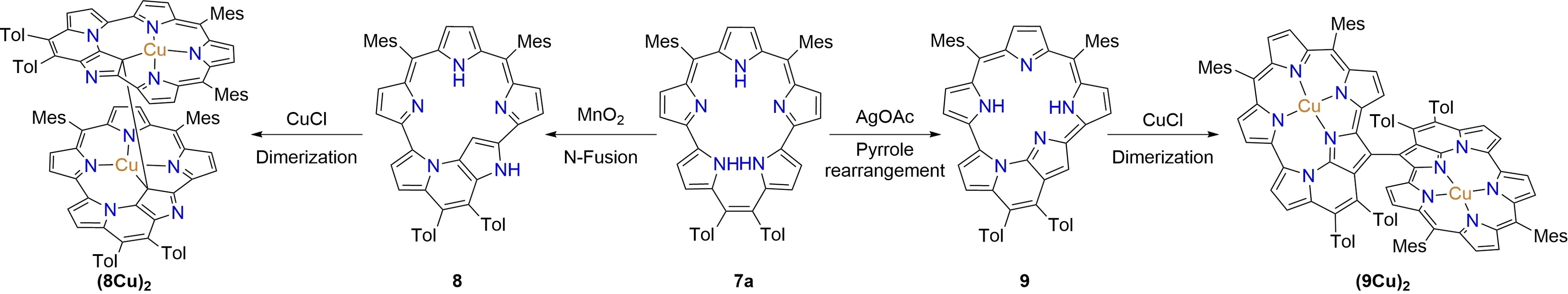
52. J. Chen, L. Liu, Y. Rao, L. Xu, M. Zhou, B. Yin, S. Shimizu, D. Shimizu, A. Osuka, J. Song
“[22]Pentaphyrins(2.0.1.1.0) Displaying N-Fusion, Pyrrole-Rearrangement, and Dimerization Reactions Upon Oxidation and Metalation”
Angew. Chem. Int. Ed. 2024, 63, e202407340. [DOI: 10.1002/anie.202407340]
-
51. H. Hamamoto, D. Shimizu, K. Matsuda
“peri-Benzo-Diindenotetracenyl: Helically π-Extended Allyl Radical with Robust Stability”
Chem. Eur. J. 2024, 30, e202401353. [DOI: 10.1002/chem.202401353]
*Highlighted in Synfacts, 2024, 20, 1040.

-
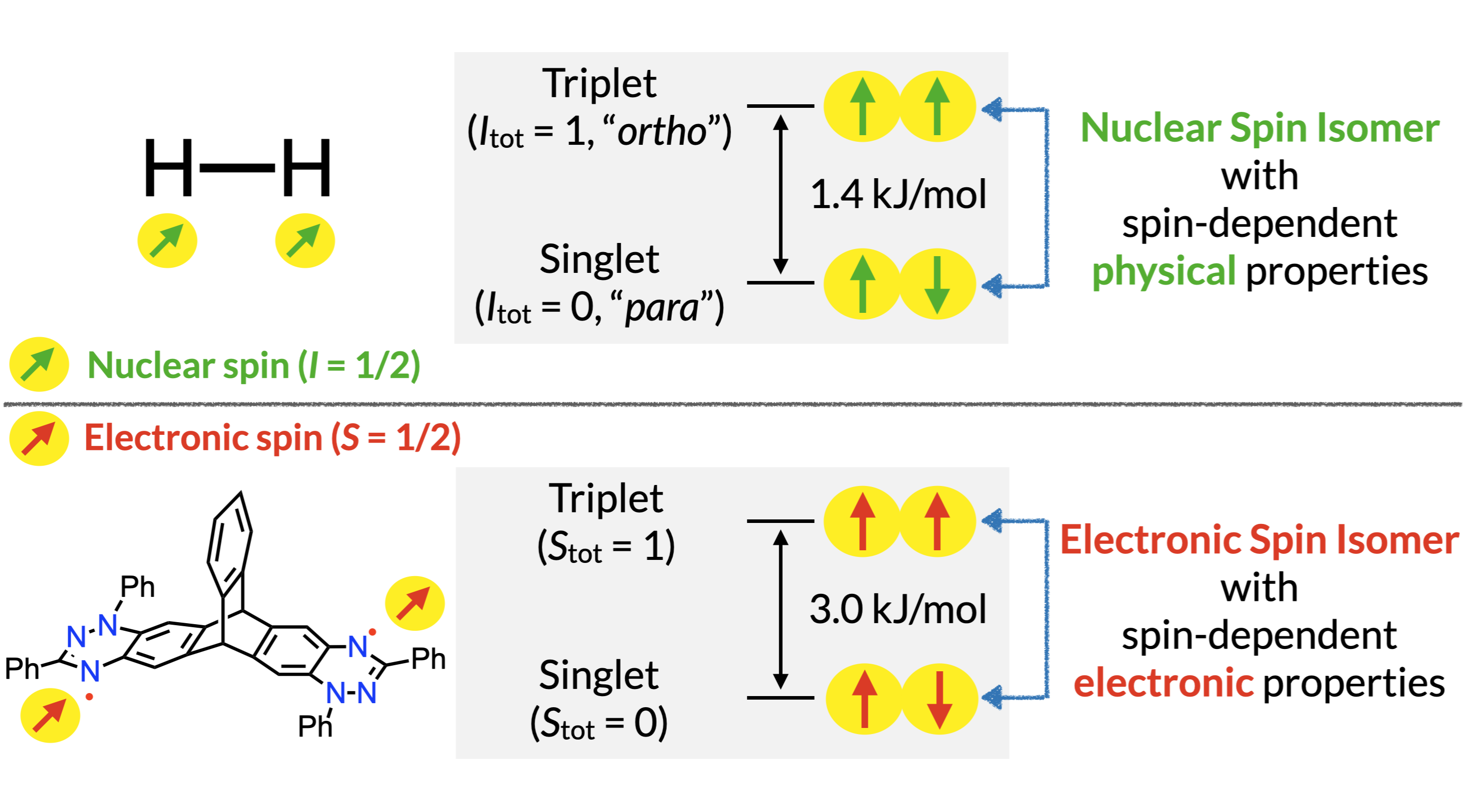
50. D. Shimizu, H. Sotome, H. Miyasaka, K. Matsuda
“Optically Distinguishable Electronic Spin-isomers of a Stable Organic Diradical”
ACS Cent. Sci. 2024, 10, 890-898. [DOI: 10.1021/acscentsci.4c00284] (Open access)
*Press Release [KyotoU] [OsakaU]
*Highlighted in Chem-Station
-
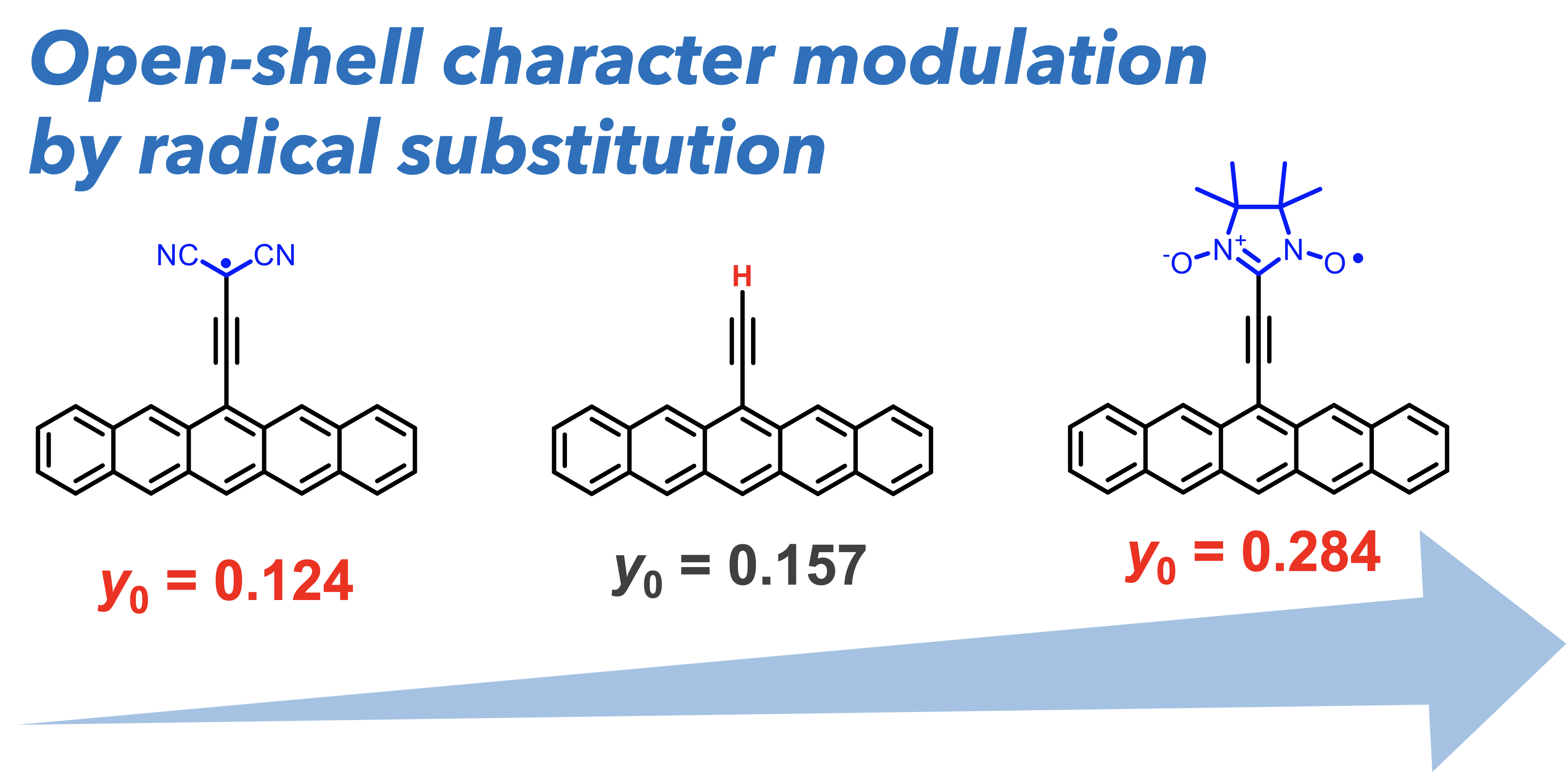
49. T. Shinozuka, D. Shimizu, K. Matsuda
“Theoretical investigation on the effect of radical substituents on the open-shell character of polycyclic aromatic hydrocarbon”
New J. Chem. 2024, 48, 8683-8689. [DOI: 10.1039/D4NJ00555D] (Open access)
-
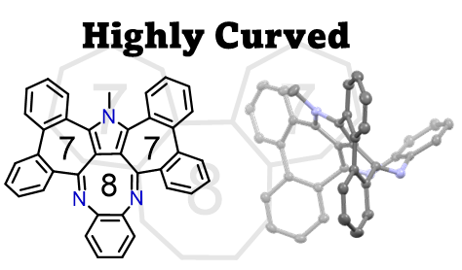
48. M. Hisada, D. Shimizu, K. Matsuda
“Synthesis and properties of doubly diphenylene-fused benzopyrrolo[1,4]diazocine with a [7-8-7] successive ring-fused structure”
Chem. Lett. 2024, 53, upae021. [DOI: 10.1093/chemle/upae021]
-
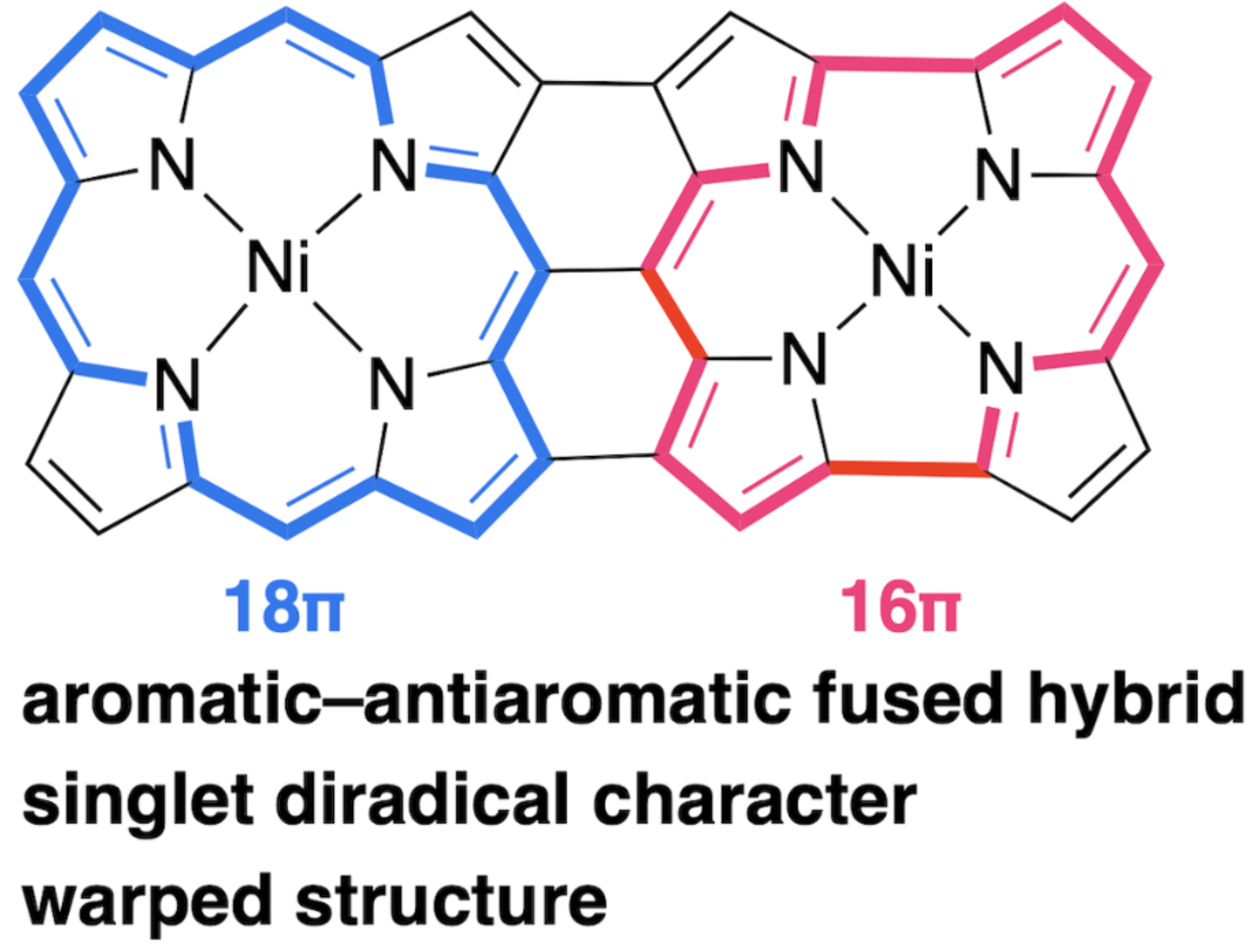
47. K. Wang, S. Ito, S. Ren, D. Shimizu, N. Fukui, R. Kishi, Q. Liu, A. Osuka, J. Song, H. Shinokubo
“A Triply-Linked Porphyrin−Norcorrole Hybrid with Singlet Diradical Character”
Angew. Chem. Int. Ed. 2024, 63, e202401233. [DOI: 10.1002/anie.202401233]
-
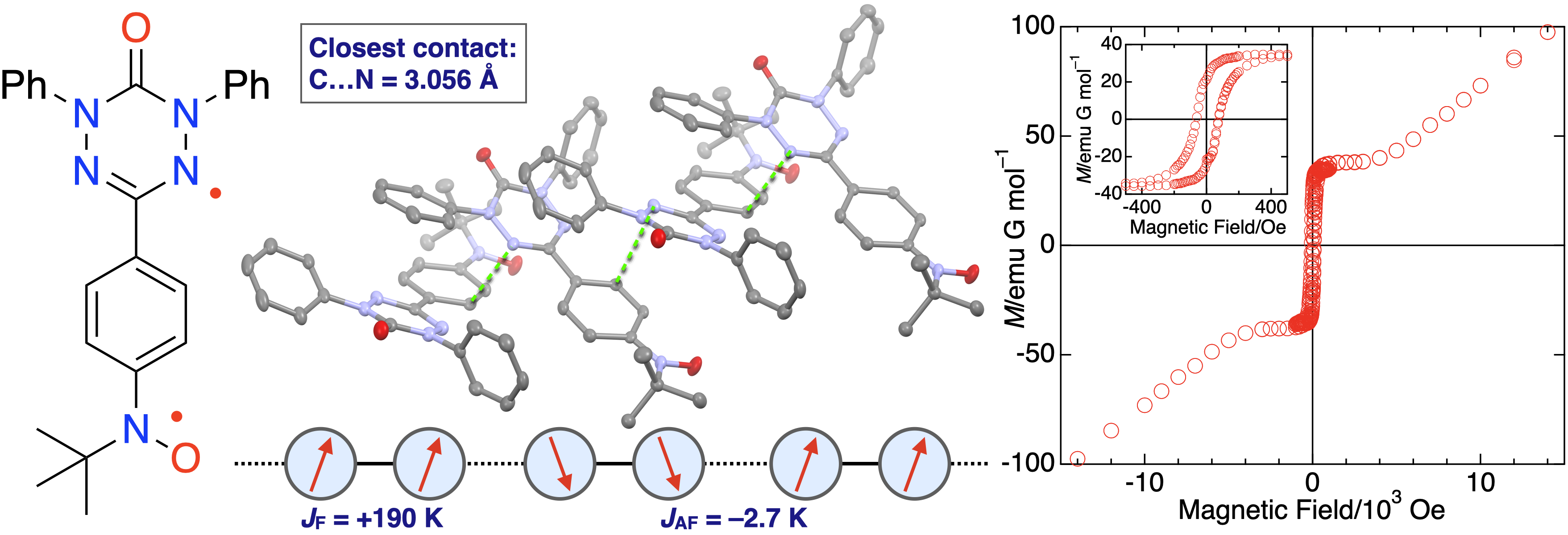
46. H. Hamamoto, D. Shimizu, K. Matsuda
“Verdazyl–Nitroxide Diradical with S = 1 Ground State: Observation of Long-Range Ordering and Haldane Gap in a Highly Isotropic S = 1 Antiferromagnetic Heisenberg Chain”
J. Phys. Chem. C 2023, 127, 21822-21828. [DOI: 10.1021/acs.jpcc.3c05584]
-
45. Y. Takeo, J. Hirano, D. Shimizu, N. Fukui, H. Shinokubo
“Effect of internal oxygen substituents on the properties of bowl-shaped aromatic hydrocarbons”
Org. Chem. Front. 2023, 10, 5895-5901. [DOI: 10.1039/D3QO01661G]

-
44. M. Kato, J. Kim, J. Oh, D. Shimizu, N. Fukui, H. Shinokubo
“Near-Infrared-Responsive Hydrocarbons Designed by π-Extension of Indeno[1,2,3,4-pgra]perylene at the 1,2,12-Positions”
Chem. Eur. J. 2023, 29, e202300249. [DOI: 10.1002/chem.202300249]
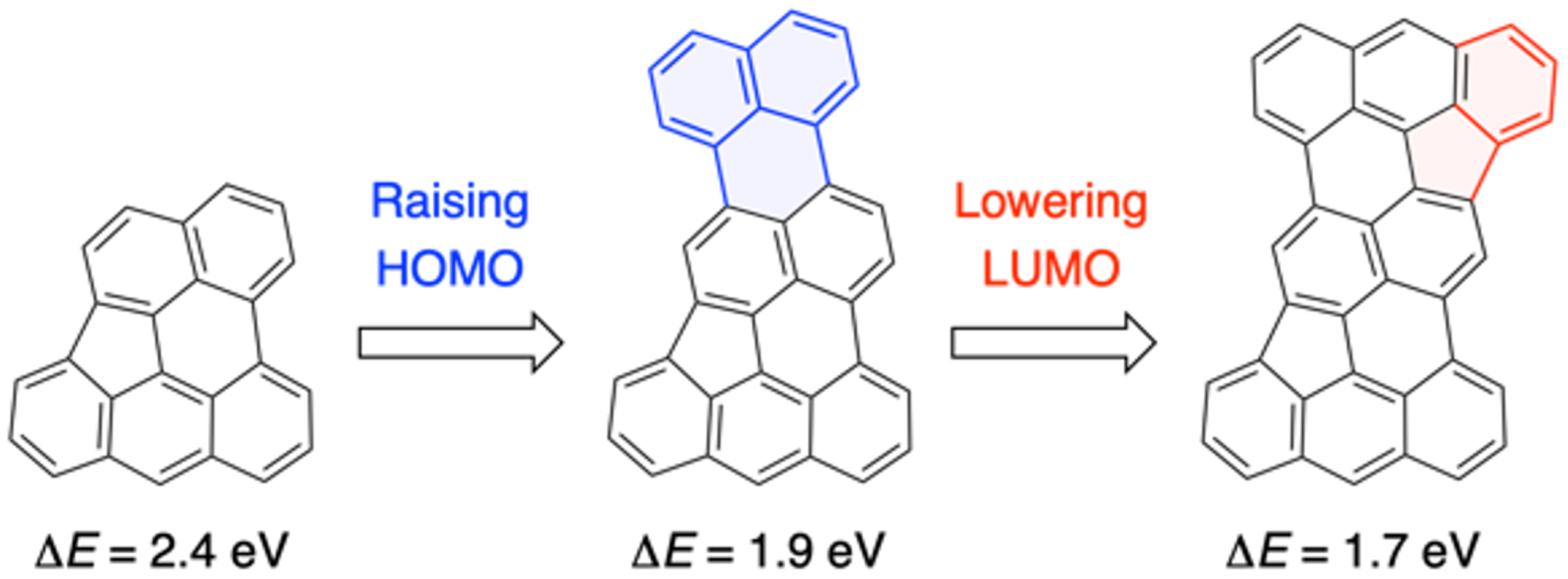
-
43. W. Deng, Y. Liu, D. Shimizu, T. Tanaka, A. Nakai, Y. Rao, L. Xu, M. Zhou, A. Osuka, J. Song
“Facile Formation of Stable Neutral Radicals and Cations from [22]Smaragdyrin BF2 Complexes”
Chem. Eur. J. 2023, 29, e202203484. [DOI: 10.1002/chem.202203484]
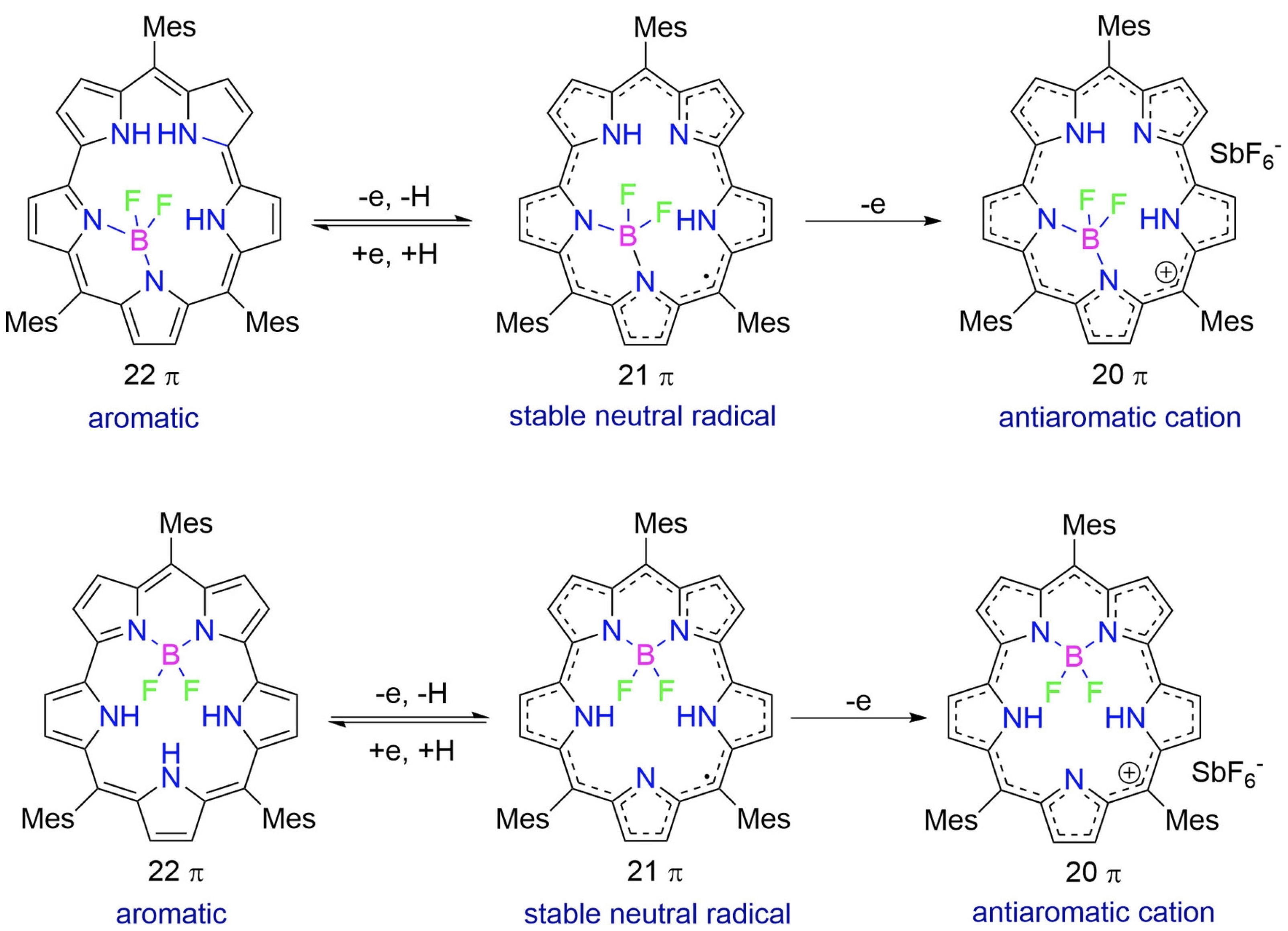
-
42. M. Hisada, D. Shimizu, K. Matsuda
“π-Expansion of 2,3,6,7-Tetraazanaphthalene with Two Embedded Heptagons: Highly Twisted Structure and Lone-Pair/π* Interaction in the Crystal”
Org. Lett. 2022, 24, 3707-3711. [DOI: 10.1021/acs.orglett.2c01345]
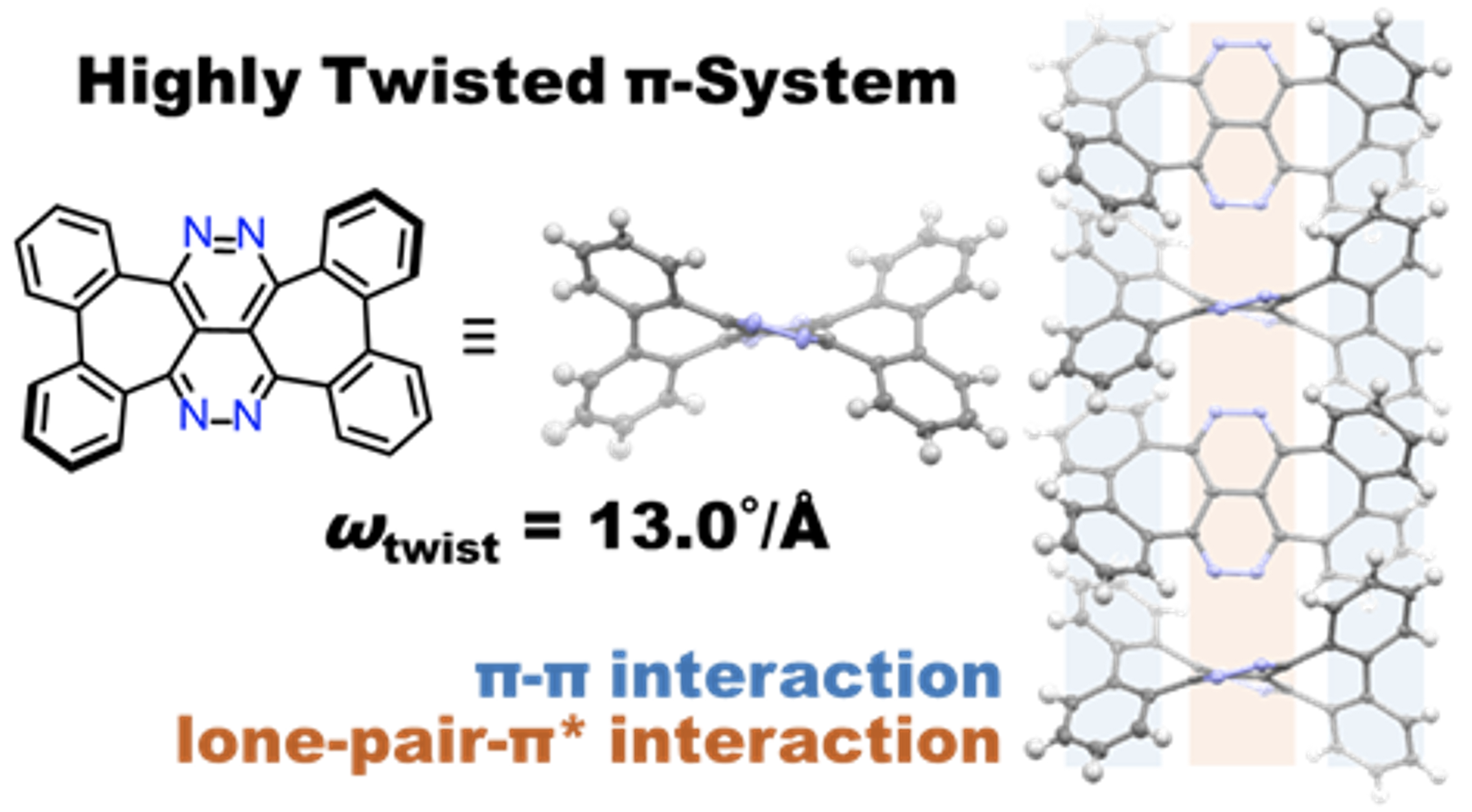
-
41. M. Hisada, D. Shimizu, K. Matsuda
“Heptagon-Embedded π-Expanded Thieno- and N-Methylpyrrolo-Pyridazines with Substantial Out-of-Plane Dipole Moment”
J. Org. Chem. 2022, 87, 9034-9043. [DOI: 10.1021/acs.joc.2c00709]
(Preprint: ChemRxiv, 10.26434/chemrxiv-2022-cnpr2).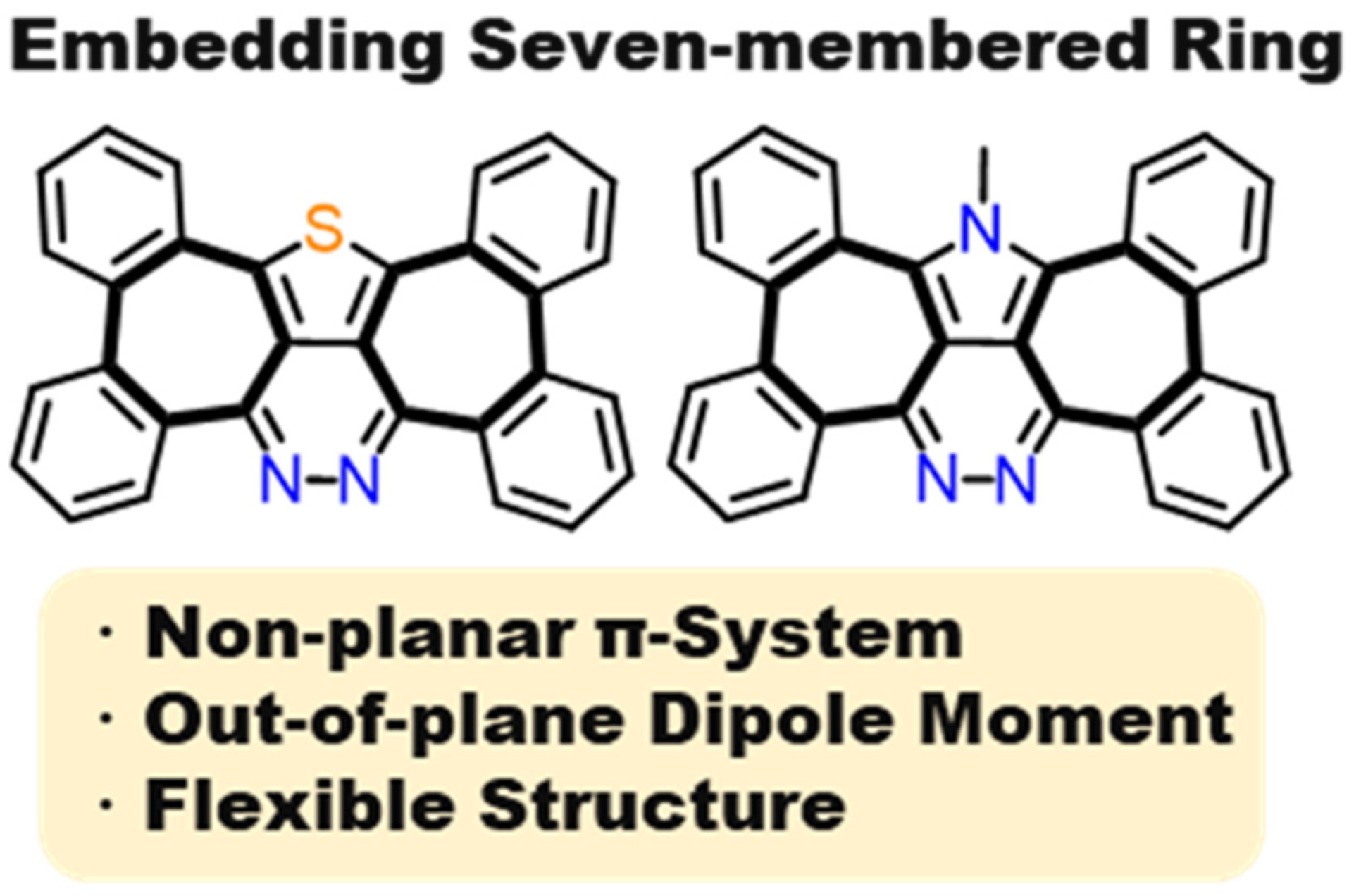
-
40. R. Yasui, D. Shimizu, K. Matsuda
“Large Enhancement of Single Molecular Conductance of Molecular Wire through a Radical Substituent”
Chem. Eur. J. 2022, 28, e202104242. [DOI: 10.1002/chem.202104242]
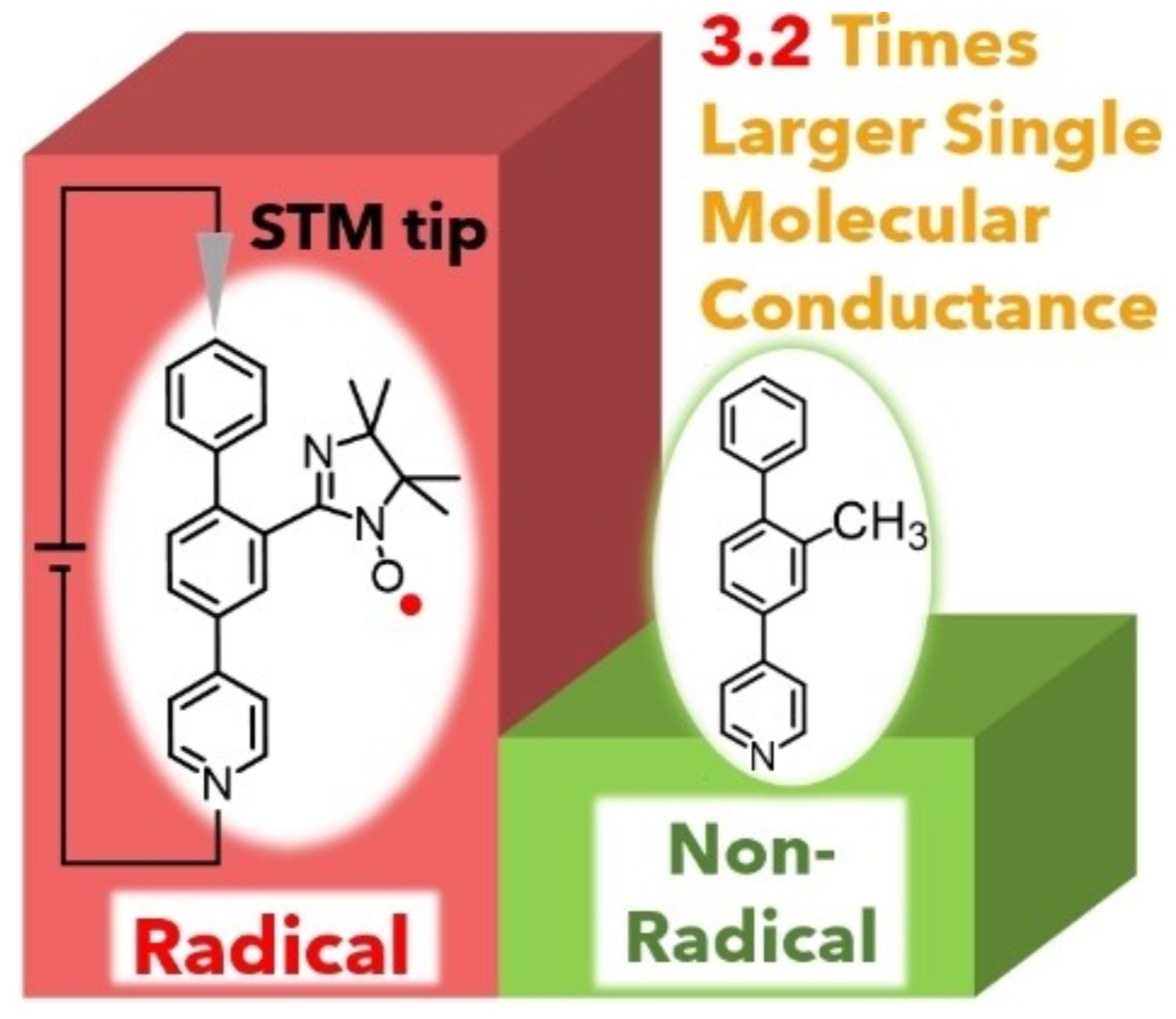

-
39. Y. Nakakuki, T. Hirose, H. Sotome, M. Gao, D. Shimizu, R. Li, J.-y. Hasegawa, H. Miyasaka, K. Matsuda
“Doubly linked chiral phenanthrene oligomers for homogeneously π-extended helicenes with large effective conjugation length”
Nat. Commun. 2022, 13, 1475. [DOI: 10.1038/s41467-022-29108-8] (Open access)
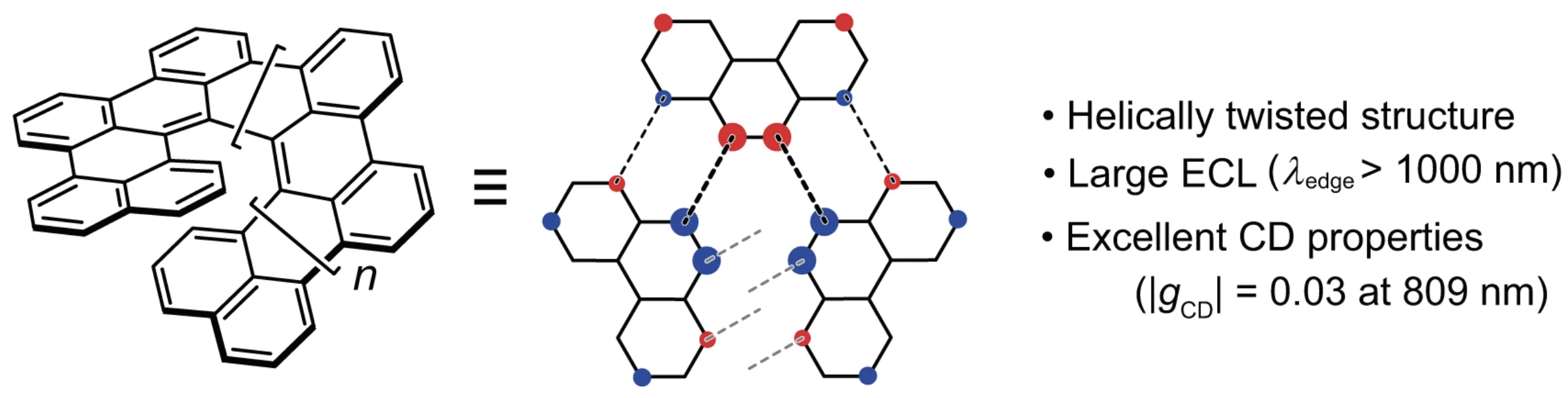
-
38. H. He, Z. Ye, D. Shimizu, I. Sumra, Y. Zhang, Z. Liang, Y. Zeng, L. Xu, A. Osuka, Z. Ke, H.-W. Jiang
“Formation of Stable Ni(III) N-Confused Porphyrins Aided by 3-Ethoxy Group”
Chem. Eur. J. 2022, 28, e202103272. [DOI: 10.1002/chem.202103272]
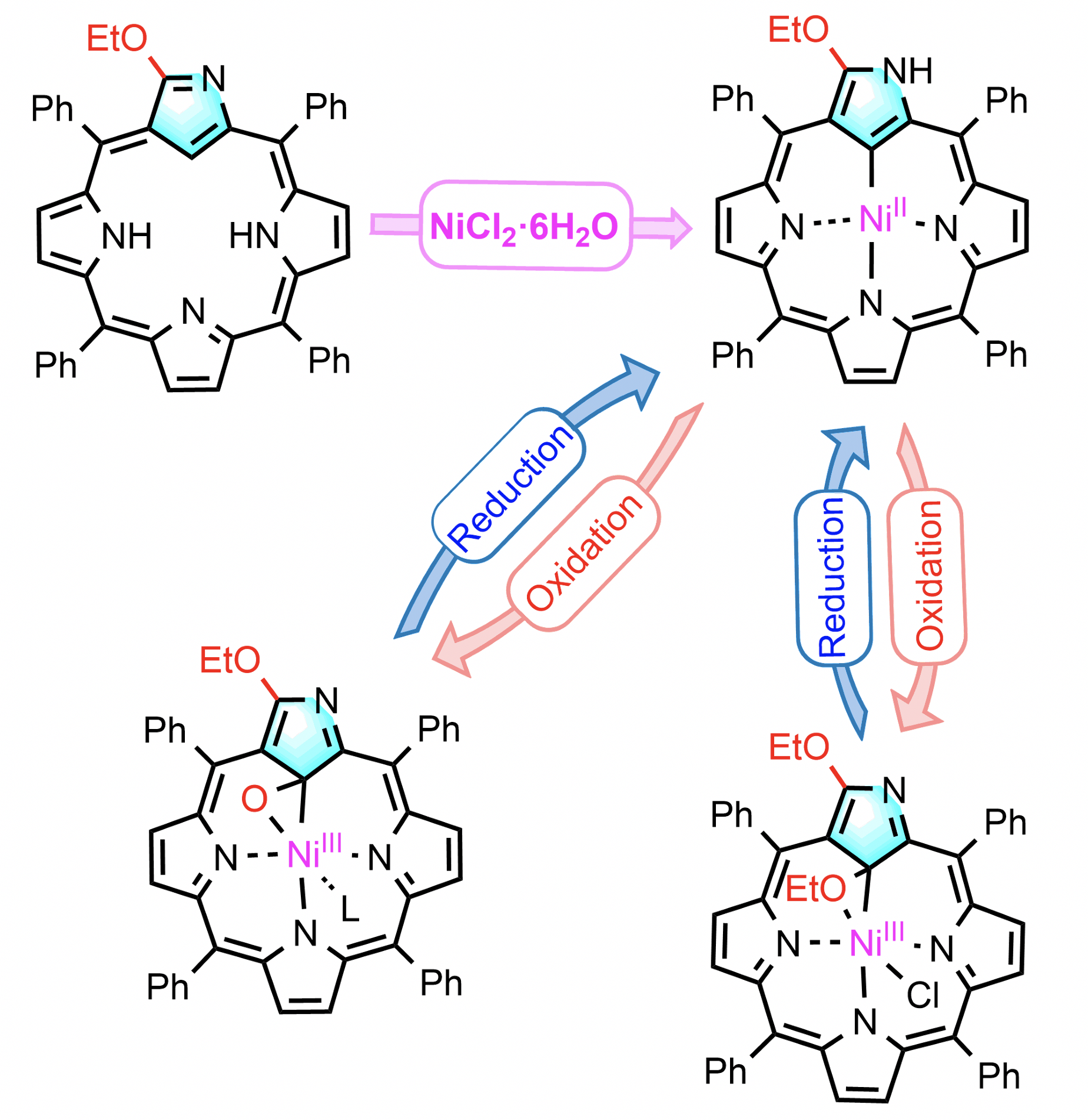
-
37. T. Shinozuka, S. Nishizawa, D. Shimizu, K. Matsuda
“Evaluation of electron transport capability of armchair graphene nanoribbons (AGNRs) by calculating exchange interaction between terminally attached radicals”
Chem. Phys. Lett. 2021, 780, 138923. [DOI: 10.1016/j.cplett.2021.138923]
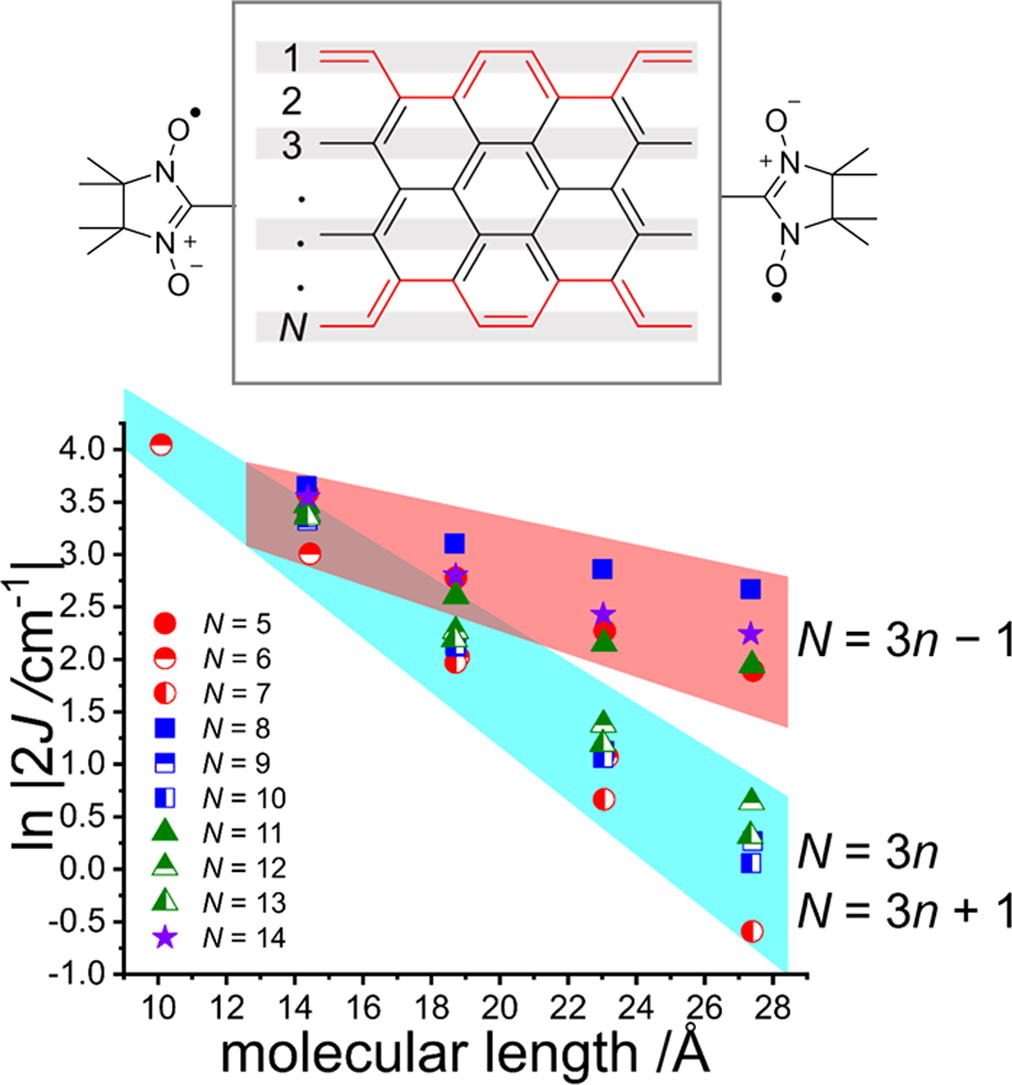
-
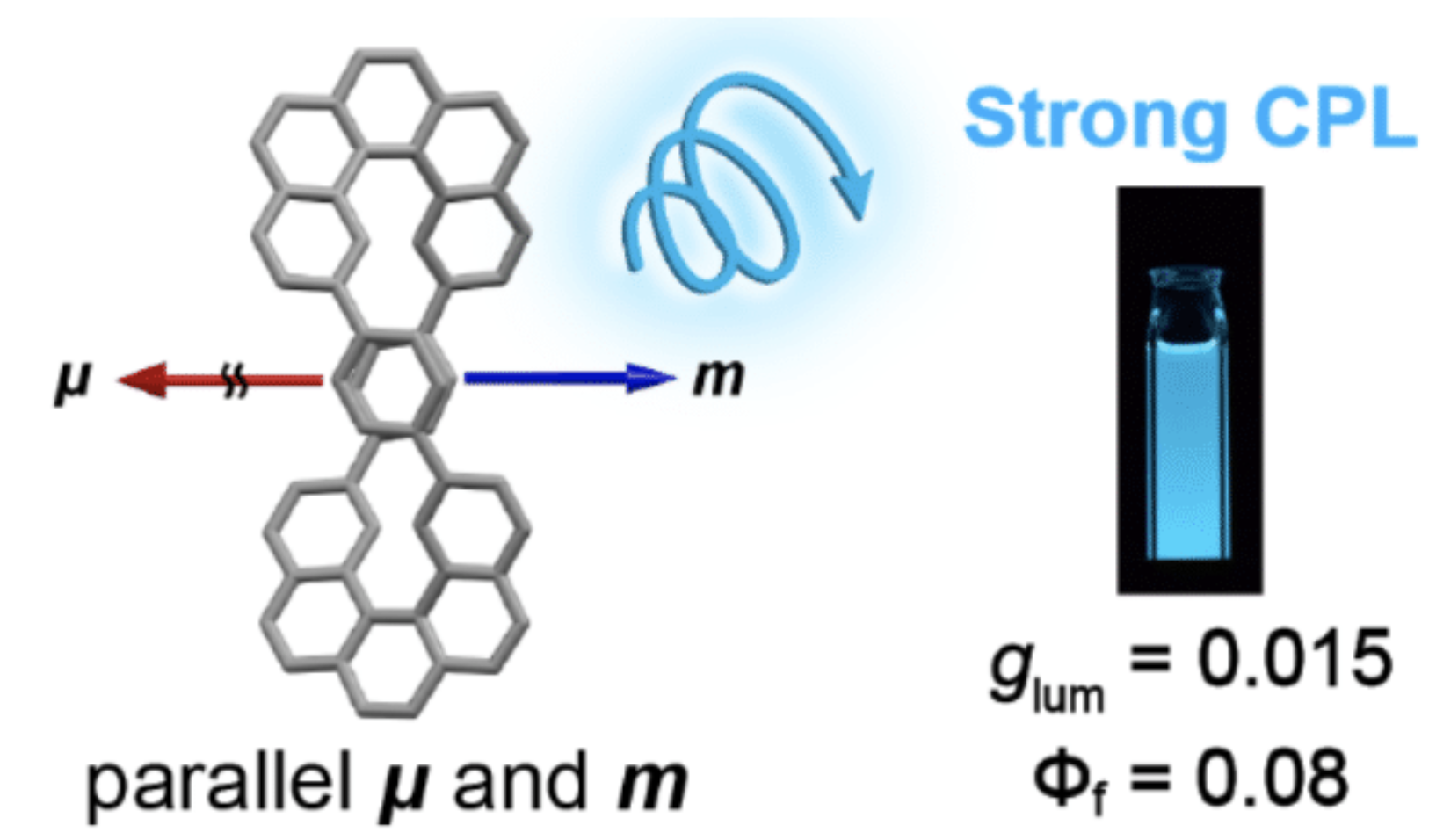
36. H. Kubo, T. Hirose, D. Shimizu, K. Matsuda
“Donor-Acceptor Type [5]Helicene Derivative with Strong Circularly Polarized Luminescence”
Chem. Lett. 2021, 50, 804-807. [DOI: 10.1246/cl.200913]
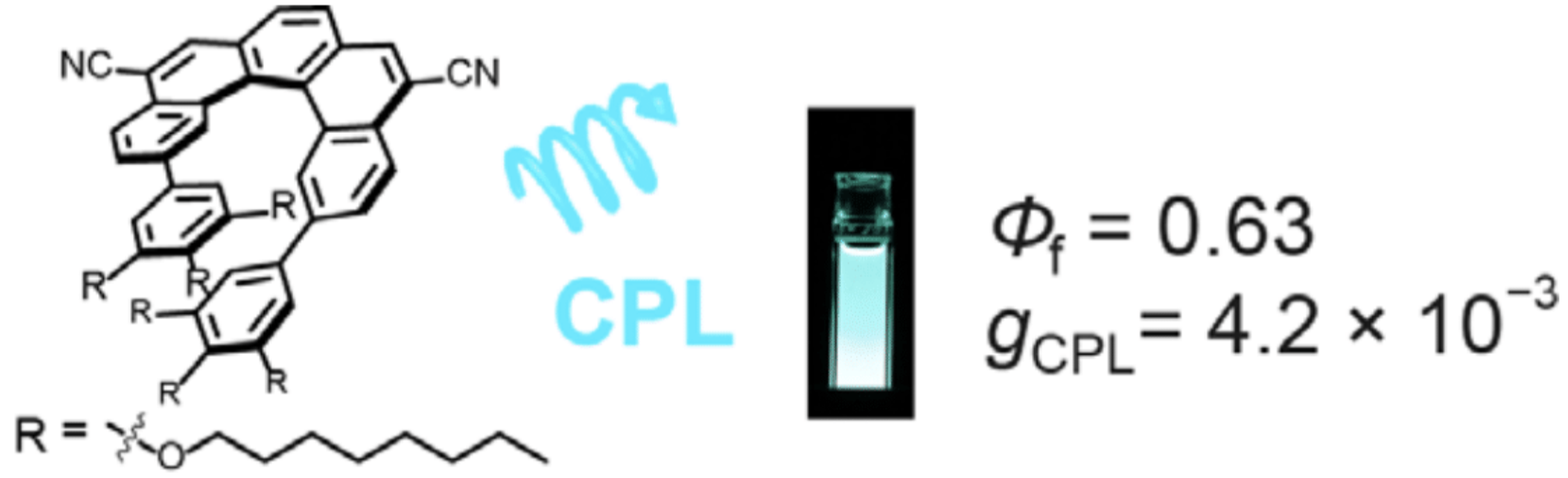
-
35. H. Kubo, D. Shimizu, T. Hirose, K. Matsuda
“Circularly Polarized Luminescence Designed from Molecular Orbit-als: A Figure-Eight-Shaped [5]Helicene Dimer with D2 Symmetry”
Org. Lett. 2020, 22, 9276-9281. [DOI: 10.1021/acs.orglett.0c03506]
-
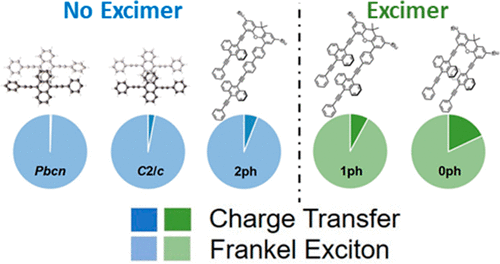
34.Y. J. Bae, D. Shimizu, J. D. Schultz, G. Kang, J. Zhou, G. C. Schatz, A. Osuka, M. R. Wasielewski
“Balancing Charge Transfer and Frenkel Exciton Coupling Leads to Excimer Formation in Molecular Dimers: Implications for Singlet Fission”
J. Phys. Chem. A 2020, 124, 8478-8487. [DOI: 10.1021/acs.jpca.0c07646]
-
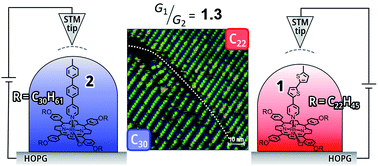
33. T. Iizuka, D. Shimizu, K. Matsuda
“STM apparent height measurements of molecular wires with different physical length attached on 2-D phase separated templates for evaluation of single molecular conductance”
RSC Adv. 2020, 10, 22054-22057. [DOI: 10.1039/D0RA04484A]
-
32. S. Ooi, B. Adinarayana, D. Shimizu, T. Tanaka, and A. Osuka
“Stable meso-meso Linked 2NH-Corrole Radical Dimers as a Key Intermediate to Corrole Tape”
Angew. Chem. Int. Ed. 2020, 59, 9423-9427. [DOI: 10.1002/anie.202002976]
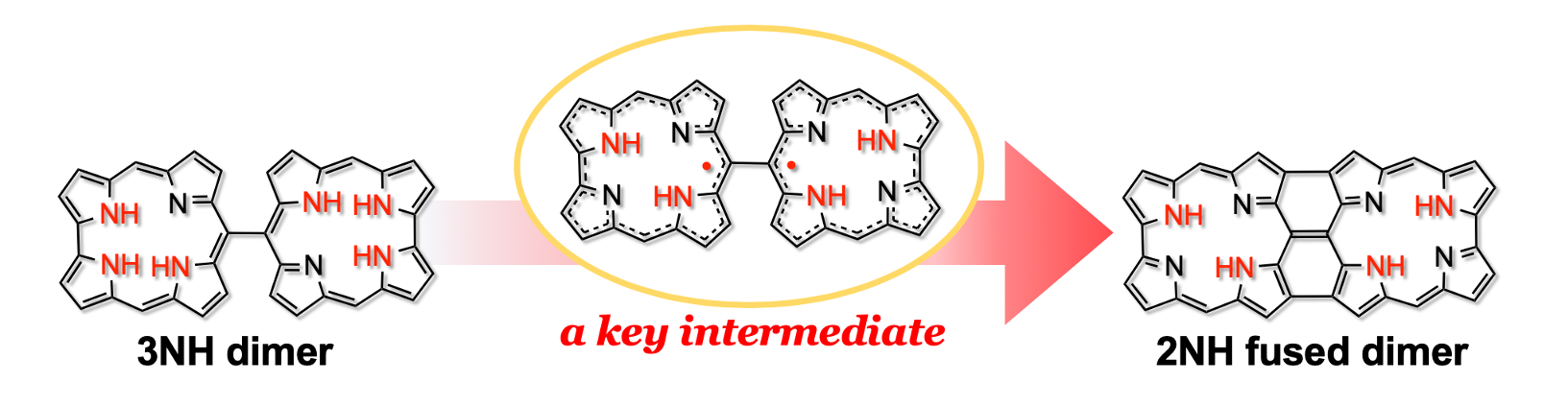
-
31. M. Izawa, T. Suito, S. Ishida, D. Shimizu, T. Tanaka, T. Mori, A. Osuka
“Figure-eight Octaphyrin Bis-Ge(IV) Complexes: Synthesis, Structures, Aromaticity, and Chiroptical Properties”
Chem. Asian J. 2020, 15, 1440-1448. [DOI: 10.1002/asia.202000159]
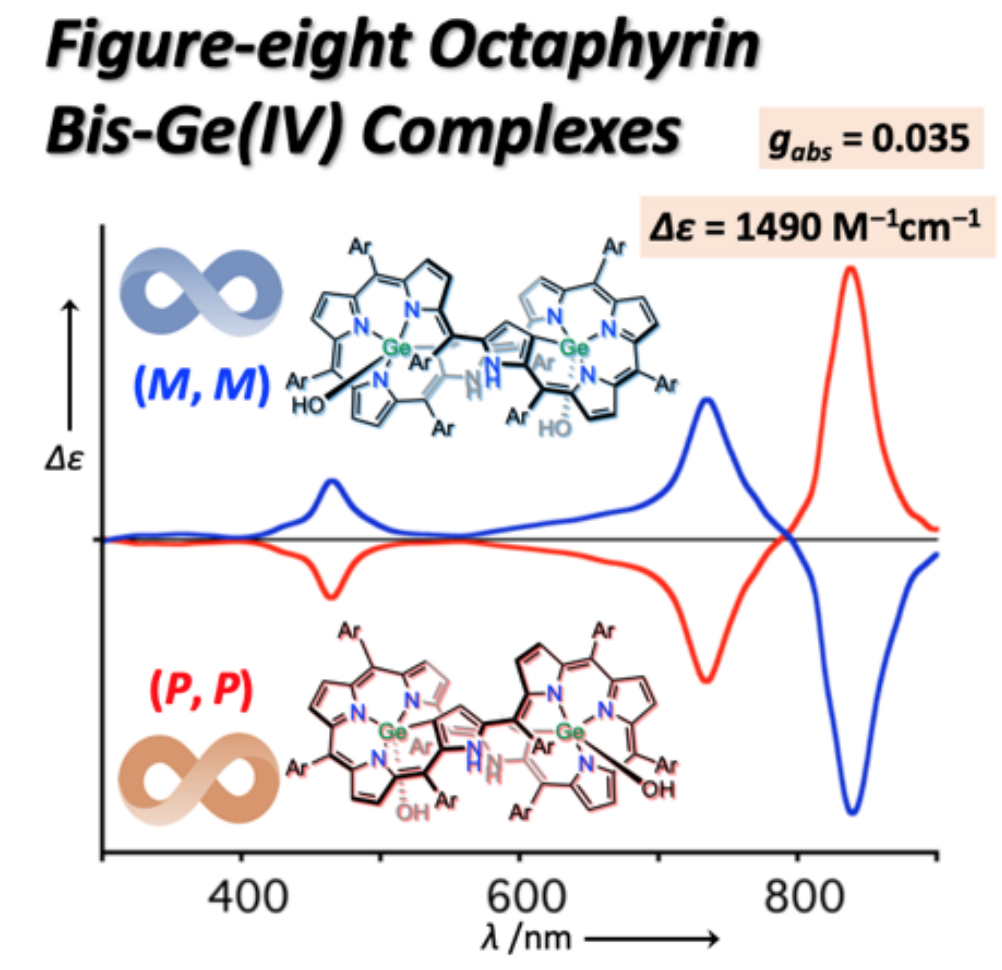
-
30. G. Lavarda, D. Shimizu, T. Torres, A. Osuka
“meso-(2-Pyridyl)-boron(III)-subporphyrin: Perimeter Iridium(III) Coordination”
Angew. Chem. Int. Ed. 2020, 59, 3127-3130. [DOI: 10.1002/anie.201914853]
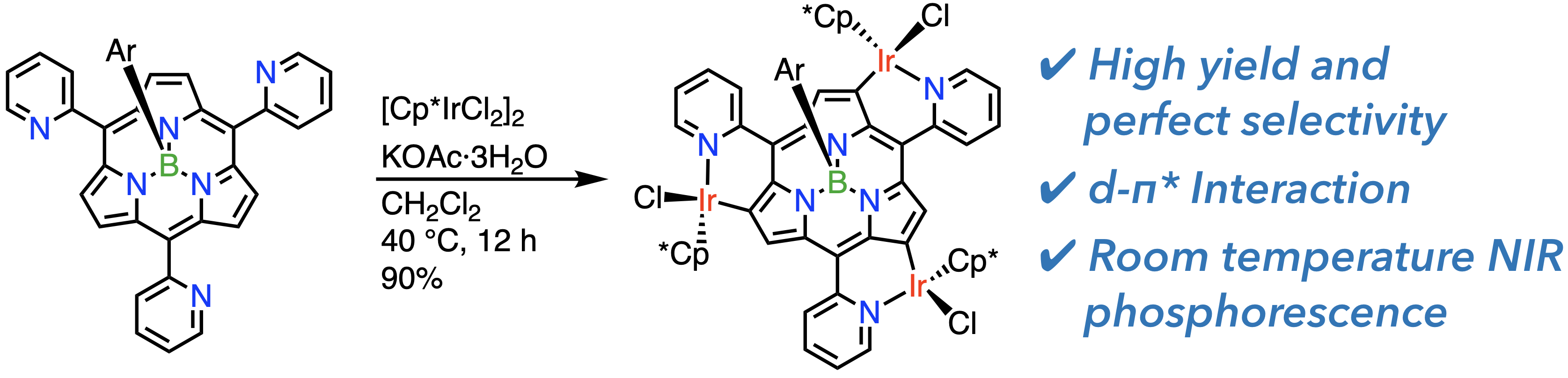
-
29. B. Adinarayana, K. Kato, D. Shimizu, K. Furukawa, T. Tanaka, A. Osuka
“Cyclophane-type Chlorin Dimers from Dynamic Covalent Chemistry of 2,18-Porphyrinyl Dicyanomethyl Diradicals”
Angew. Chem. Int. Ed. 2020, 4320-4323. [DOI: 10.1002/anie.201914480]
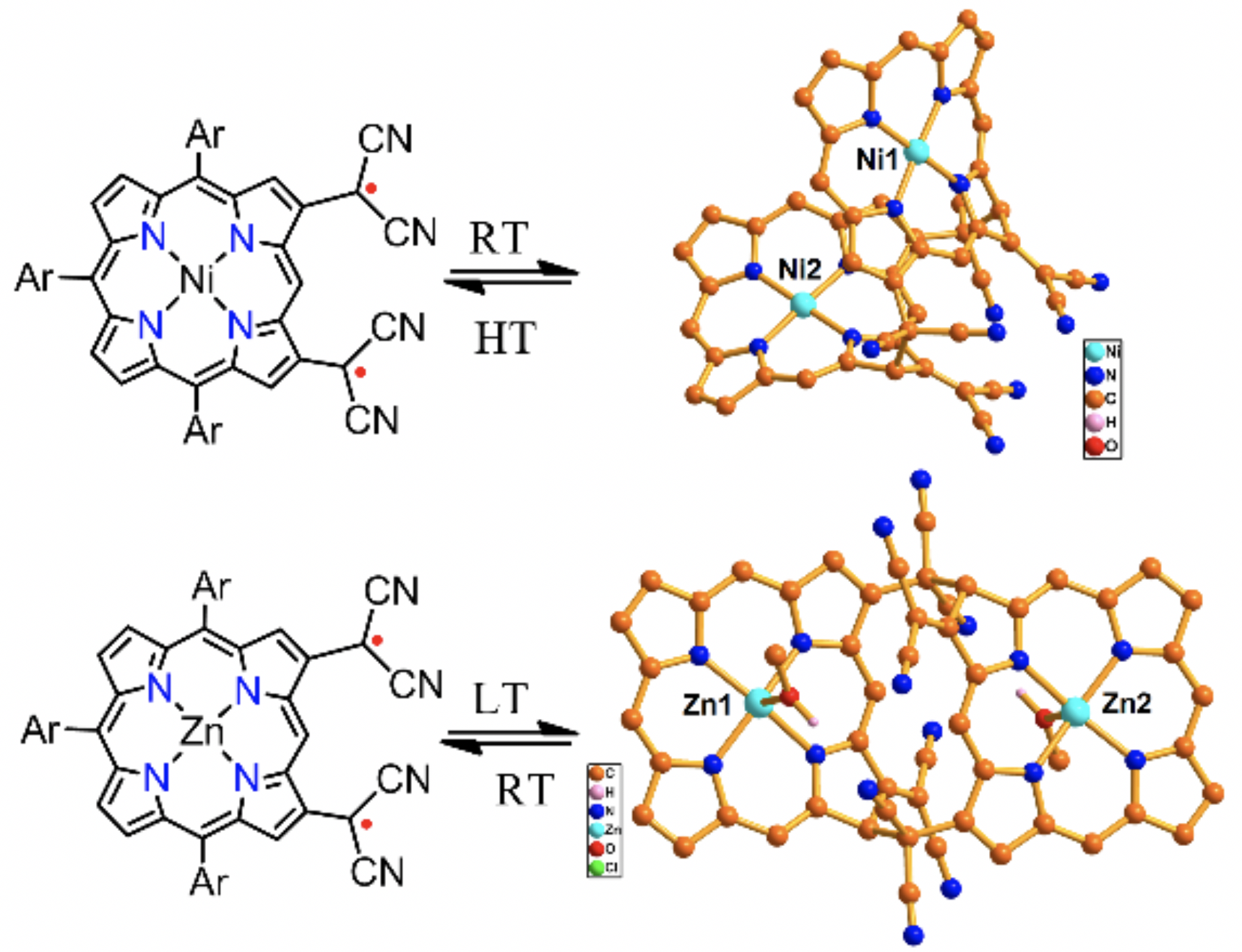
-
28. T. Yamamoto, K. Kato, D. Shimizu, T. Tanaka, A. Osuka
“Phenylene-bridged Porphyrin meso-Oxy Radical Dimers”
Chem. Asian J. 2019, 14, 4031-4034. [DOI: 10.1002/asia.201901033]
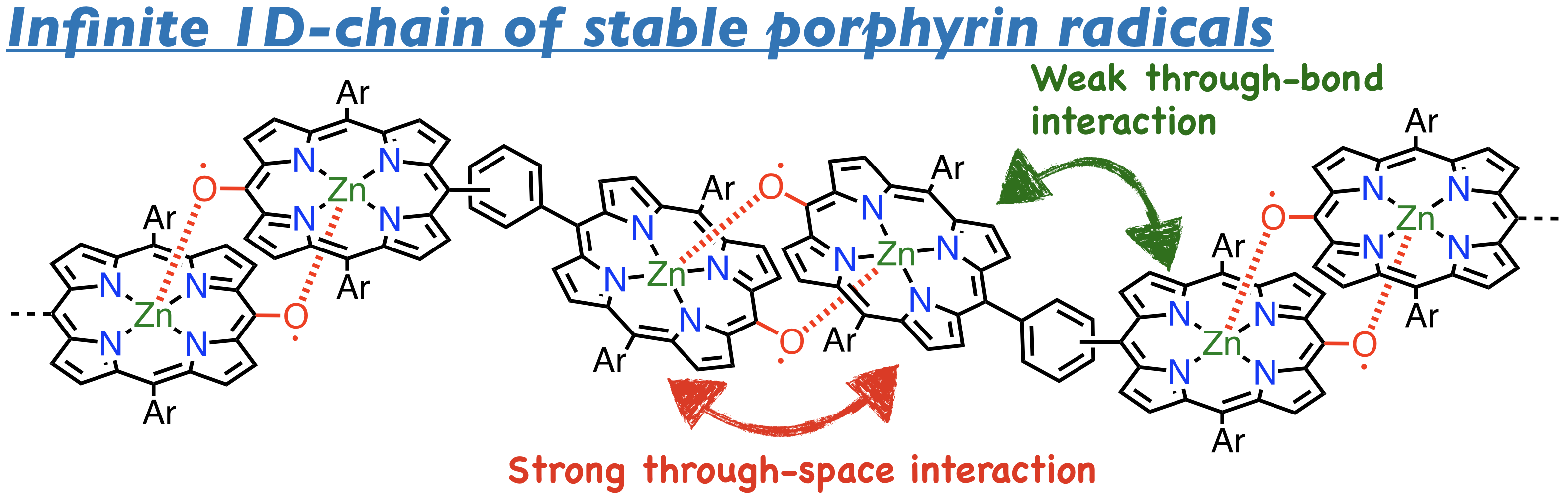
-
27. C. Schierl, W. Alex, L. M. Mateo, B. Ballesteros, D. Shimizu, A. Osuka, T. Torres, D. M. Guldi, G. Bottari
“Quadrupolar Cyclopenta[hi]aceanthrylene‐based Electron Donor‐Acceptor‐Donor Conjugates: Charge Transfer versus Charge Separation”
Angew. Chem. Int. Ed. 2019, 58, 14644-14652. [DOI: 10.1002/anie.201906206]
-
26. B. Adinarayana, D. Shimizu, K. Furukawa, A. Osuka
“Stable radical versus reversible σ-bond formation of (porphyrinyl)dicyanomethyl radicals”
Chem. Sci. 2019, 10, 6007-6012. [DOI: 10.1039/C9SC01631G] (Open access)
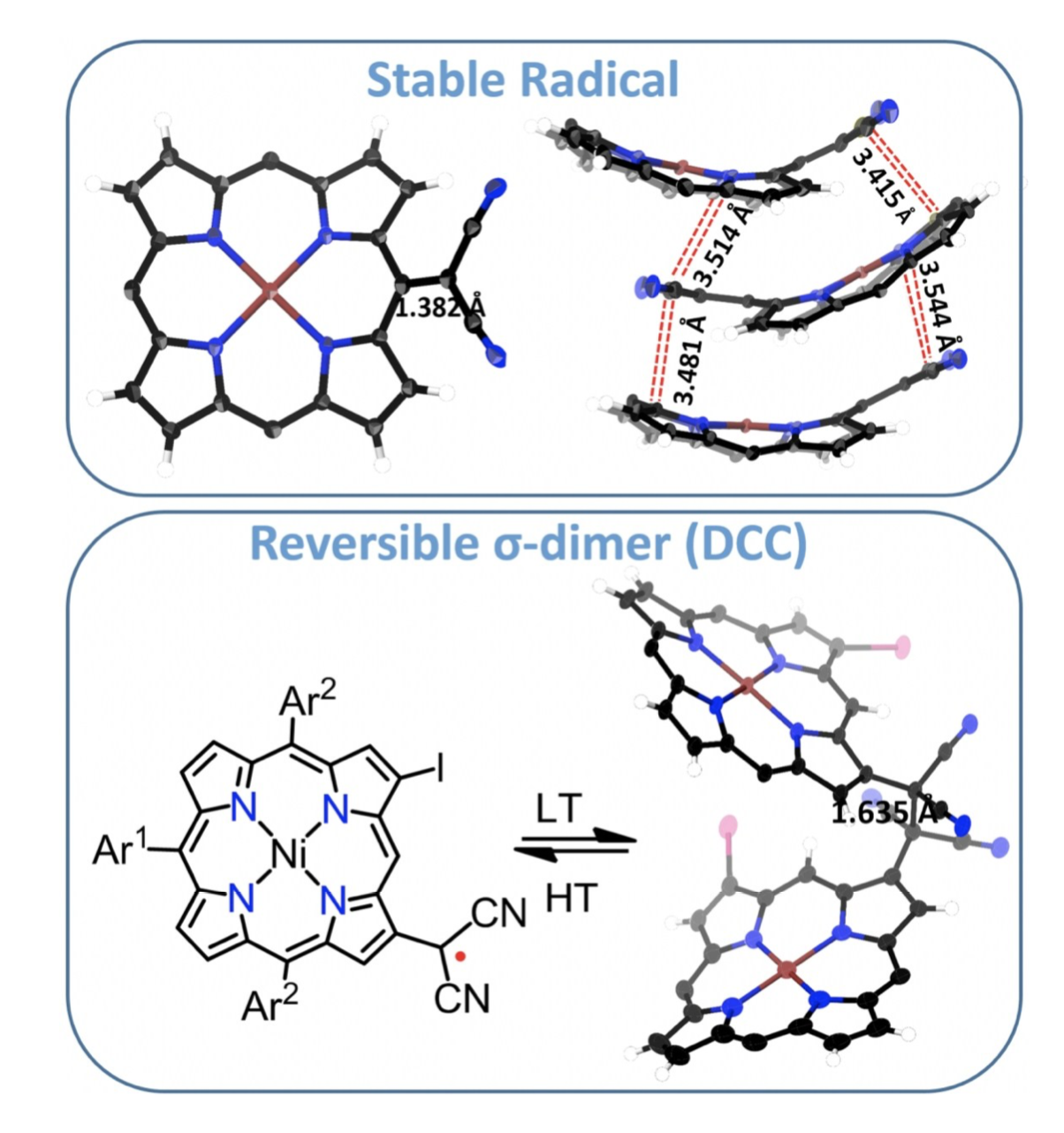
-
25. D. Shimizu, Y. Ide, T. Ikeue, A. Osuka
“Coordination-Induced Spin-State Switching of an Aminyl-Radical-Bridged Nickel(II) Porphyrin Dimer between Doublet and Sextet States”
Angew. Chem. Int. Ed. 2019, 58, 5023-5027. [DOI: 10.1002/anie.201900792]
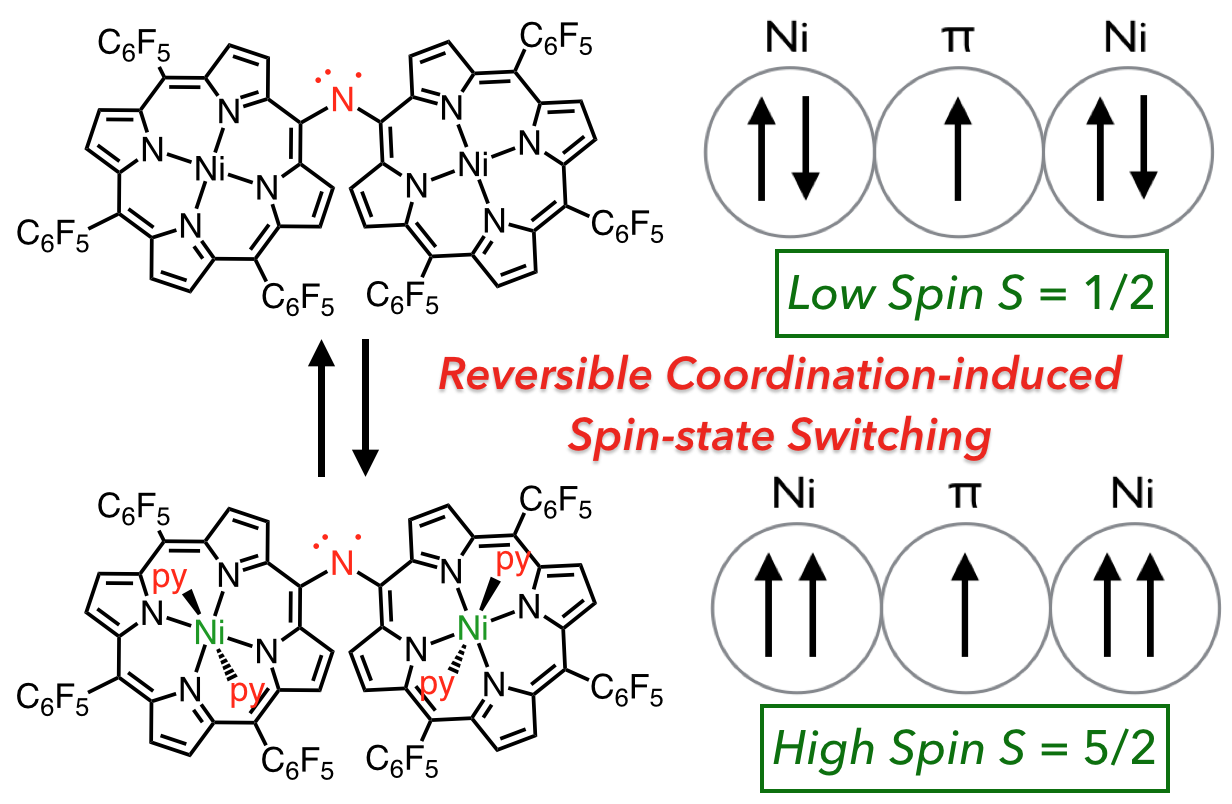
-
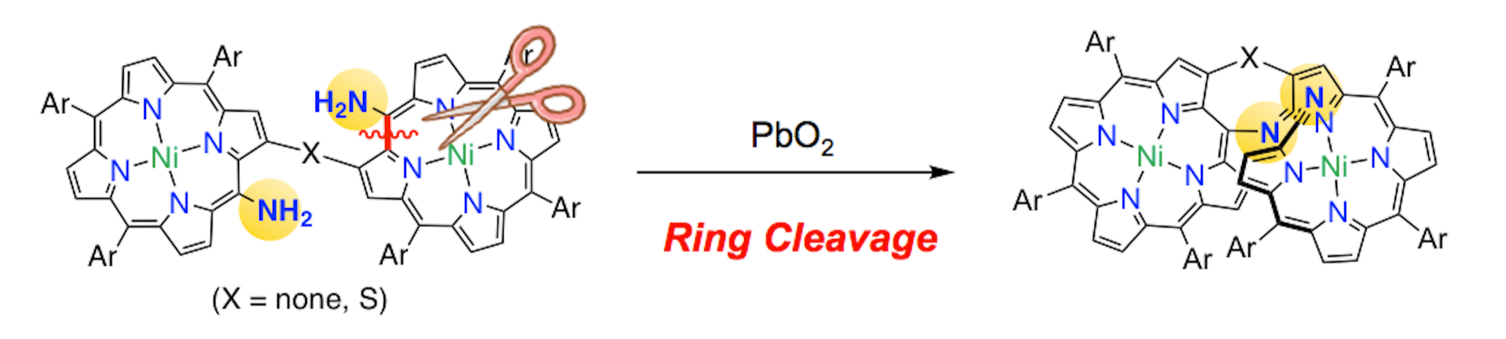
24. K. Fujimoto, D. Shimizu, T. Mori, Y. Li, M. Zhou, J. Song, A. Osuka
“Selective Formation of Helical Tetrapyrrin-fused Porphyrins by Oxidation of β-to-β Linked meso-Aminoporphyrin Dimers”
Chem. Eur. J. 2019, 25, 1711-1715. [DOI: 10.1002/chem.201805659]
-
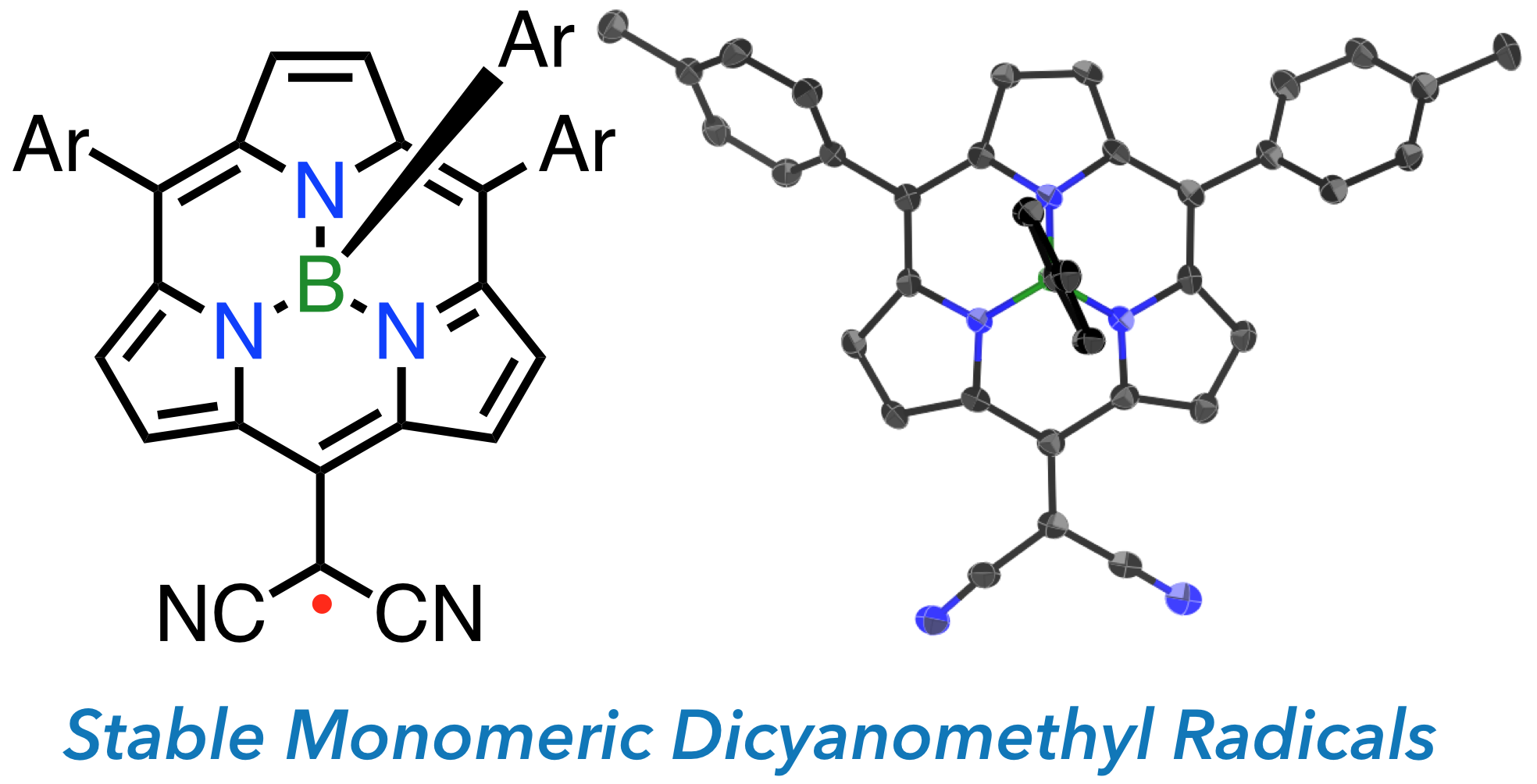
23. B. Adinarayana, D. Shimizu, A. Osuka
“Stable (BIII-Subporphyrin-5-yl)dicyanomethyl Radicals”
Chem. Eur. J. 2019, 25, 1706-1710. [DOI: 10.1002/chem.201805601]
-
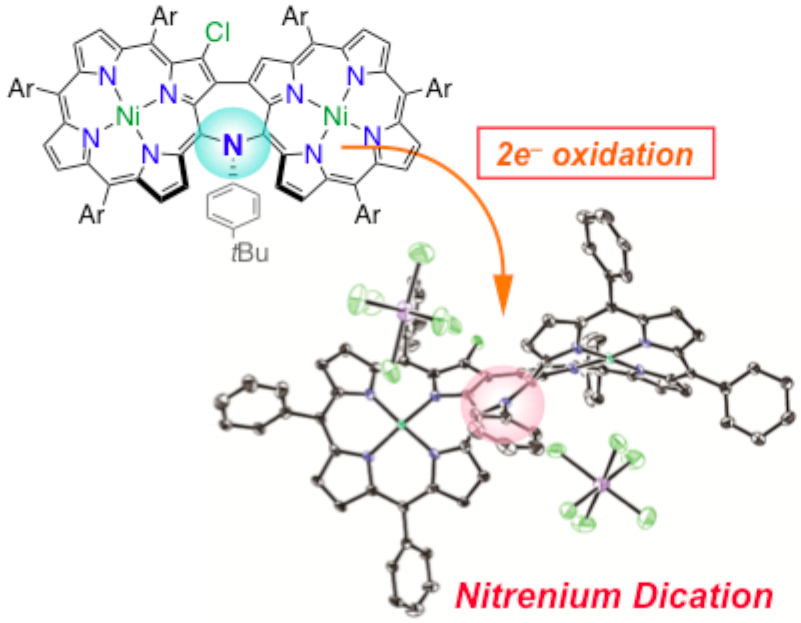
22. K. Fujimoto, D. Shimizu, A. Osuka
“Porphyrin-Stabilized Nitrenium Dication”
Chem. Eur. J. 2019, 25, 521-525. [DOI: 10.1002/chem.201805491]
-
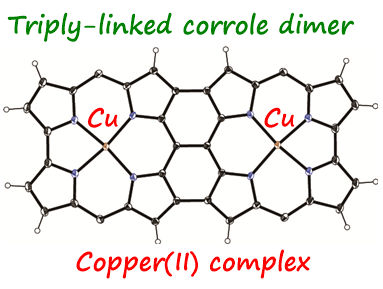
21. S. Ooi, T. Tanaka, T. Ikeue, K. Yamasumi, K Ueta, D Shimizu, M. Ishida, H. Furuta, A. Osuka
“Bis-copper(II) Complex of Triply-linked Corrole Dimer and Its Dication”
Chem. Asian J. 2019, 14, 1771-1776. [DOI: 10.1002/asia.201801467]
-
20. S. Ooi, D. Shimizu, K. Furukawa, T. Tanaka, A. Osuka
“Stable Face‐to‐Face Singlet Diradicaloids: Triply Linked Corrole Dimer Gallium(III) Complexes with Two μ‐Hydroxo‐Bridges”
Angew. Chem. Int. Ed. 2018, 57, 14916-14920. [DOI: 10.1002/anie.201810200]
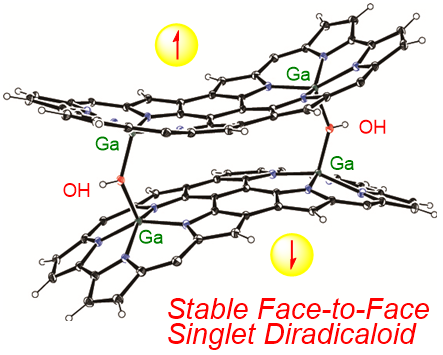
-
19. Y. Bekki, D. Shimizu, K. Fujimoto, A. Osuka
“meso-Functionalizations of BIII Subporphyrin with BIII meso-Lithiosubporphyrin”
Chem. Eur. J. 2018, 24, 12708-12715. [DOI: 10.1002/chem.201802339]
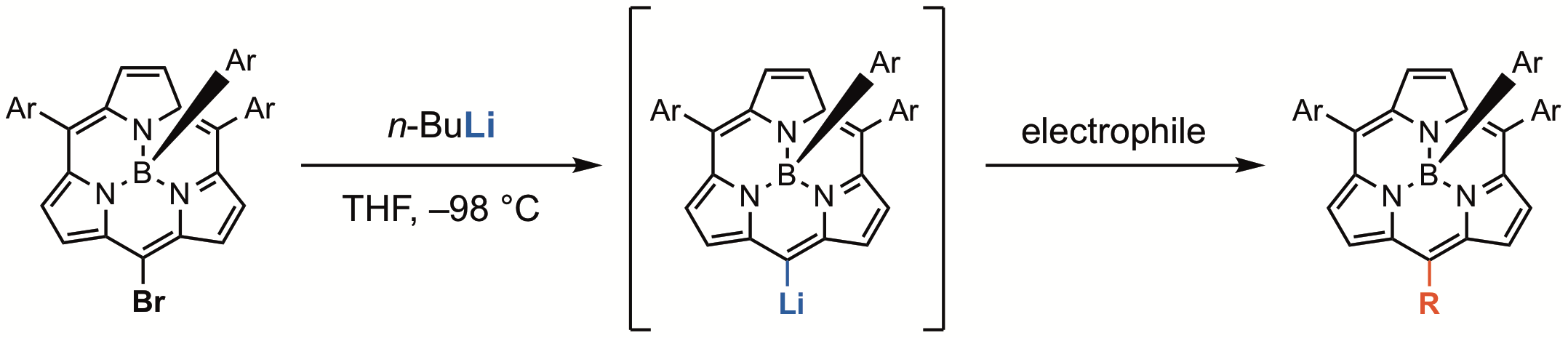
-
18. D. Shimizu, K. Fujimoto, A. Osuka
“Stable Diporphyrinyl-Aminyl Radical and Nitrenium Ion”
Angew. Chem. Int. Ed. 2018, 57, 12708-12715. [DOI: 10.1002/anie.201805385]
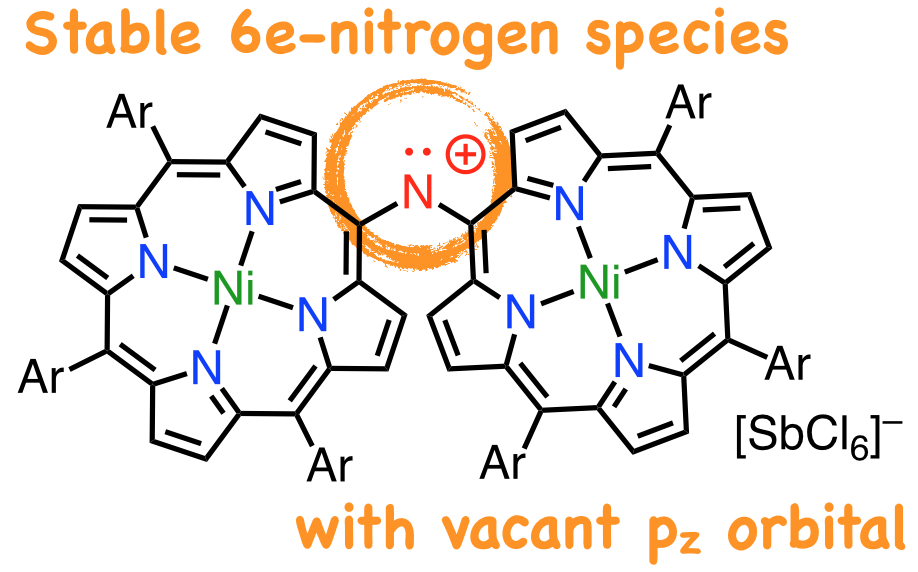
-
17. K. Kise, Y. Hong, N. Fukui, D. Shimizu, D. Kim, A. Osuka
“Diarylamine-fused Subporphyrins: Proof of Twisted Intramolecular Charge Transfer (TICT) Mechanism”
Chem. Eur. J. 2018, 24, 8306-8310. [DOI: 10.1002/chem.201801576]
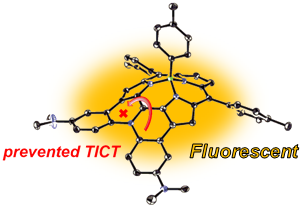
-
16. K. Kise, K. Yoshida, R. Kotani, D. Shimizu, A. Osuka
“BIII 5-Arylsubporphyrins and BIII subporphine”
Chem. Eur. J. 2018, 24, 19136-19140. [DOI: 10.1002/chem.201801491]
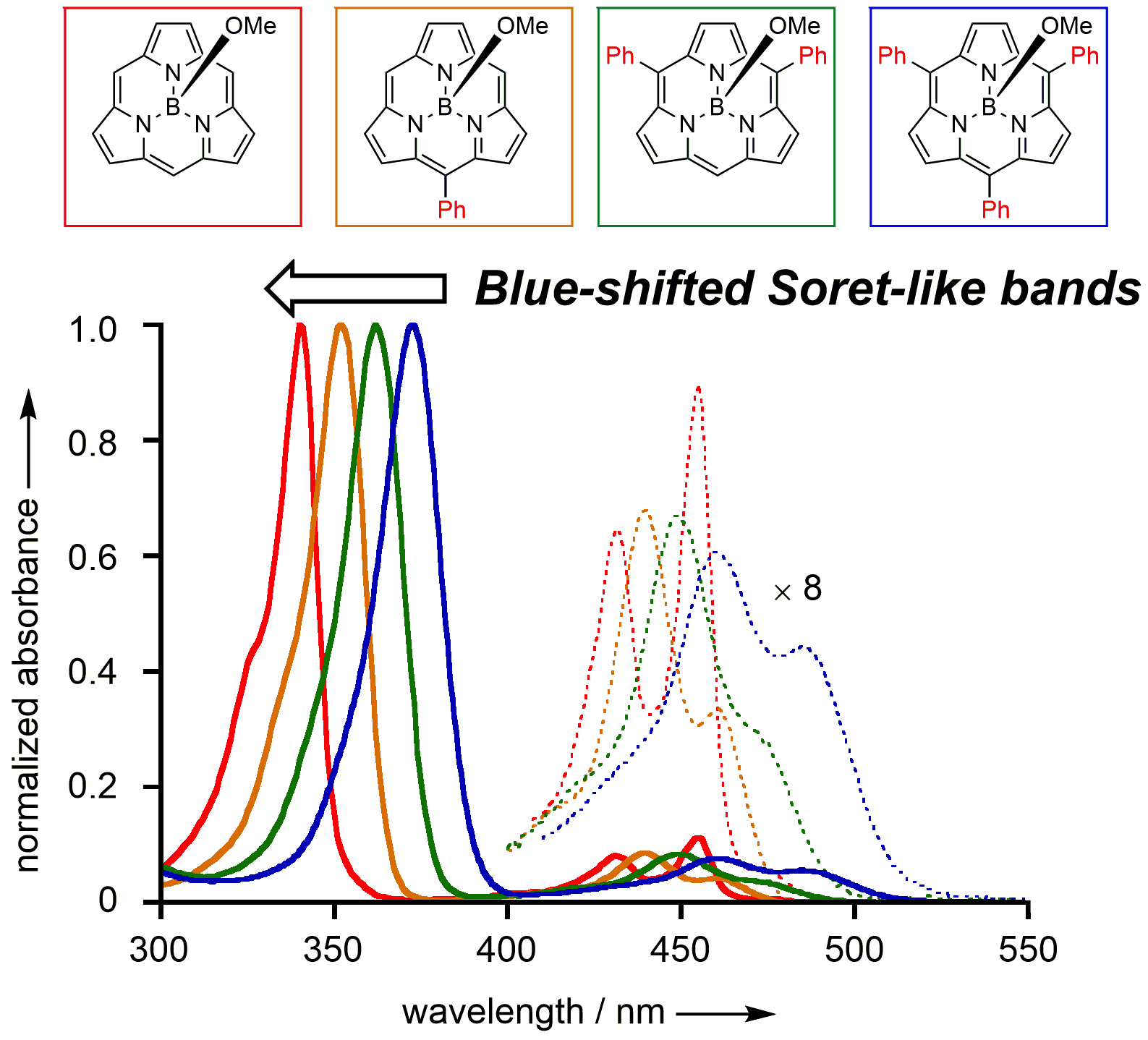
-
15. S. Ishida, J. Kim, D. Shimizu, D. Kim, A. Osuka
“Synthesis of bis-Silicon Complexes of [38]-, [37]-, and [36] Octaphyrins: Aromaticity Switch and Stable Radical Cation”
Angew. Chem. Int. Ed. 2018, 57, 5876-5880. [DOI: 10.1002/anie.201801986]
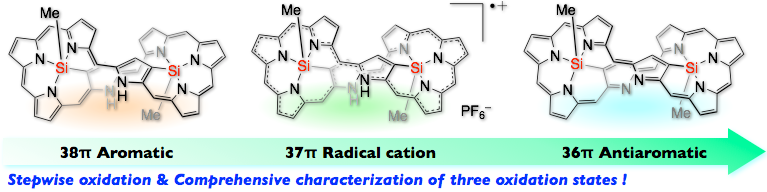
-
14. D. Shimizu, A. Osuka
“A Benzene-1,3,5-Triaminyl Radical Fused with Zn(II)-Porphyrins: Remarkable Stability and High Spin Quartet Ground State”
Angew. Chem. Int. Ed. 2018, 57, 3733-3736. [DOI: 10.1002/anie.201801080]
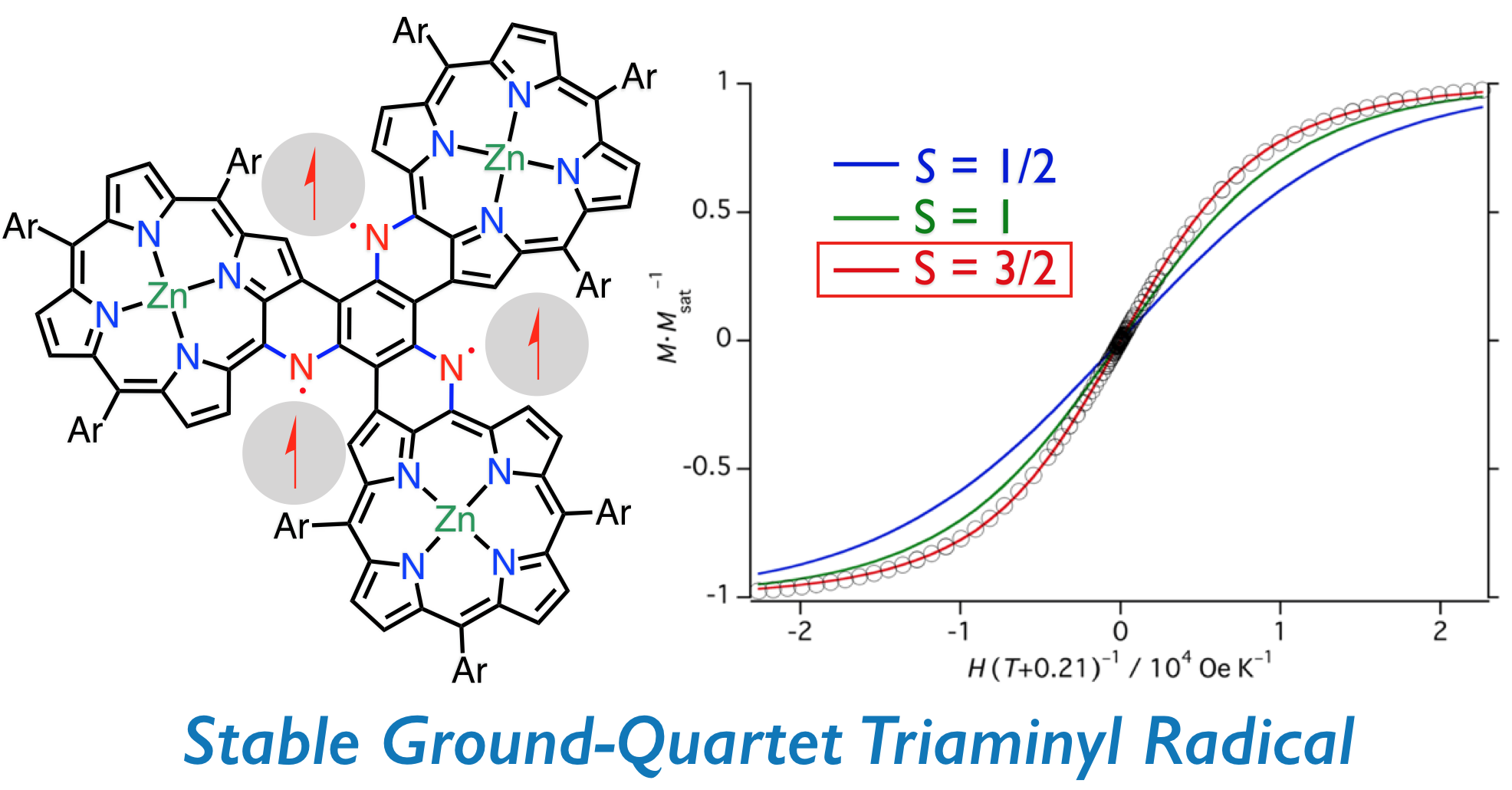
-
13. D. Shimizu, K Furukawa, A. Osuka
“Stable Subporphyrin meso-Aminyl Radicals without Resonance Stabilization by Neighboring Heteroatom”
Angew. Chem. Int. Ed. 2017, 56, 7435-7439. [DOI: 10.1002/anie.201703097]
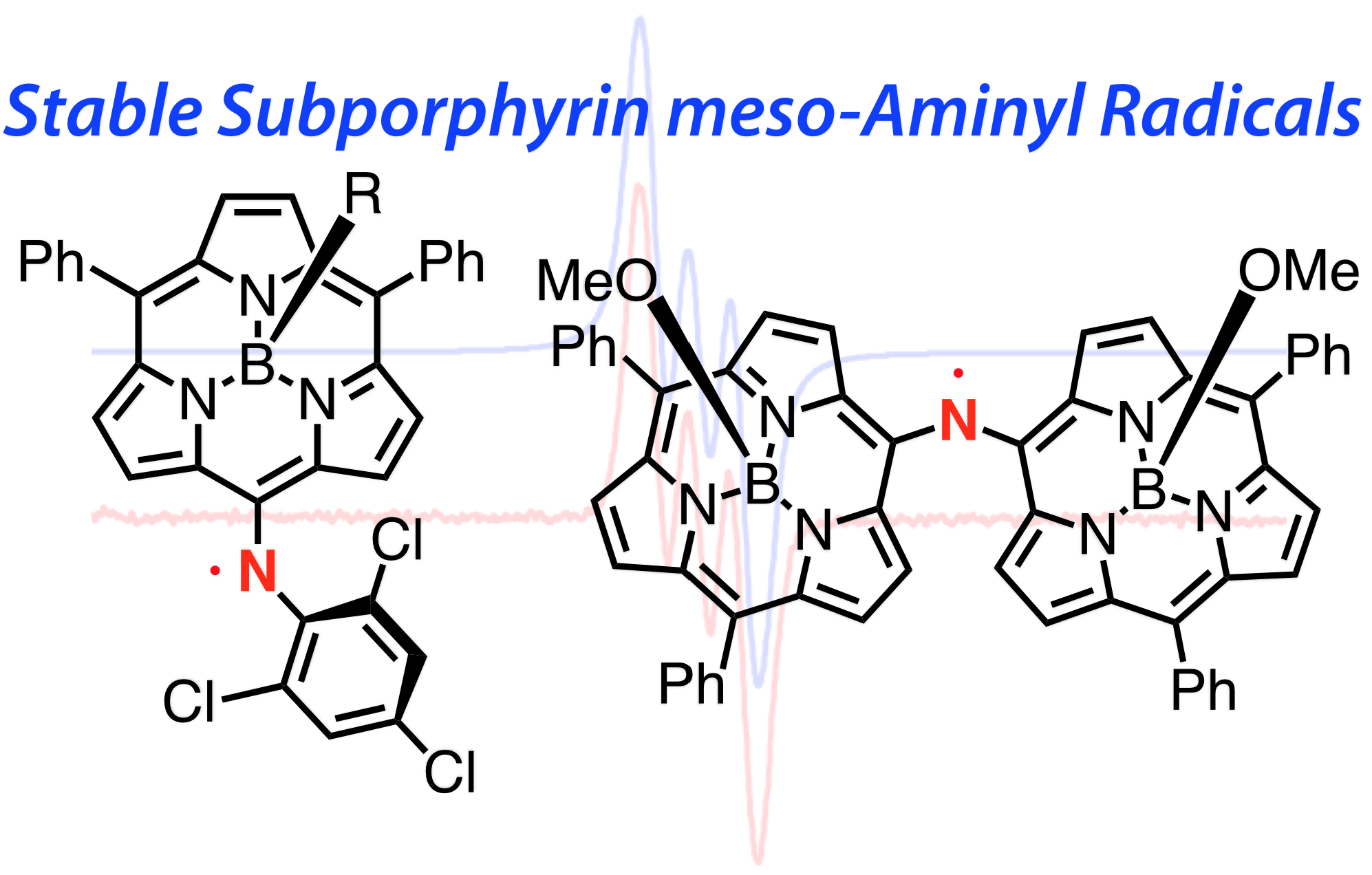
-
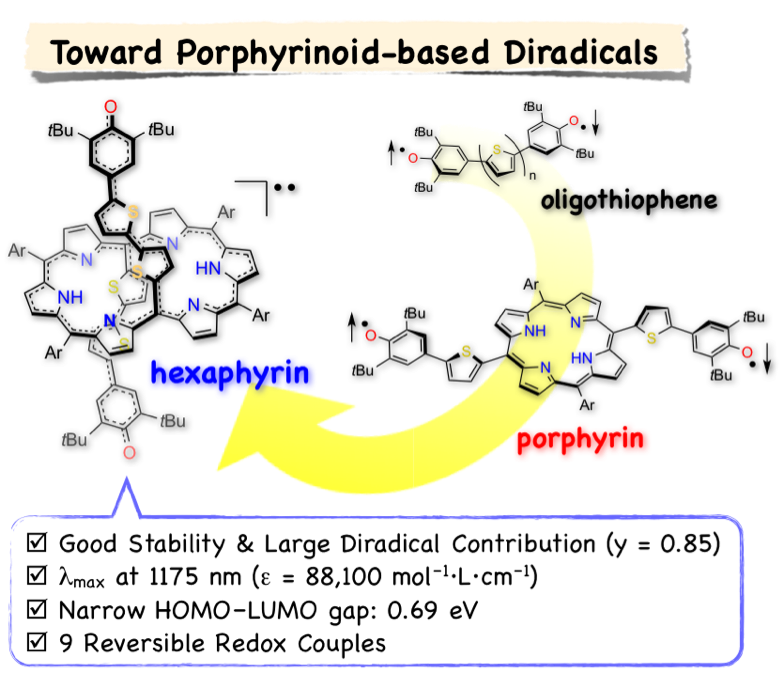
12. K. Naoda, D. Shimizu, J. O. Kim, K. Furukawa, D. Kim, A. Osuka
“Thienylquinonoidal Porphyrins and Hexaphyrins with Singlet Diradical Ground States”
Chem. Eur. J. 2017, 23, 8969-8879. [DOI: 10.1002/chem.201701355]
-
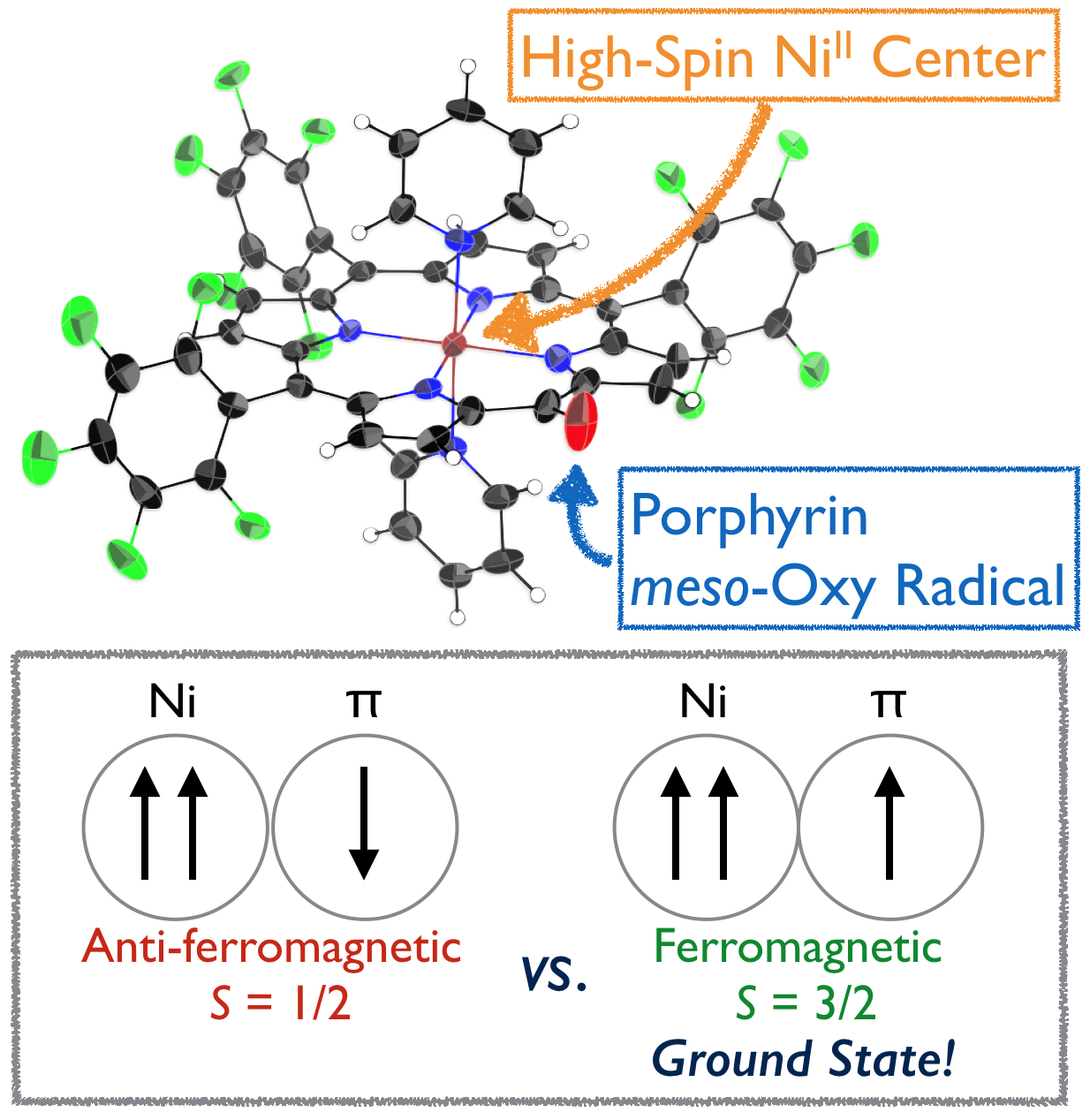
11. C. Stähler, D. Shimizu, K. Yoshida, K. Furukawa, R. Herges, A. Osuka
“Stable NiII Porphyrin meso-Oxy Radical with a Quartet Ground State”
Chem. Eur. J. 2017, 23, 7217-7200. [DOI: 10.1002/chem.201701354]
-
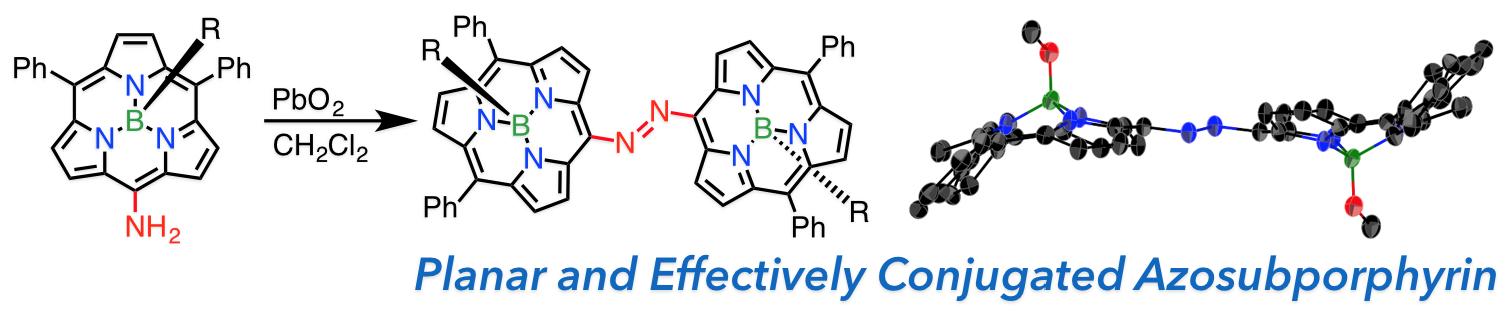
10. D. Shimizu, S.-K. Lee, D. Kim, A. Osuka
“meso-Nitro- and meso-Aminosubporphyrinatoboron(III)s and meso-to-meso-Azosubporphyrinatoboron(III)s”
Chem. Asian J. 2016, 11, 2946-2952. [DOI: 10.1002/asia.201601019]
-
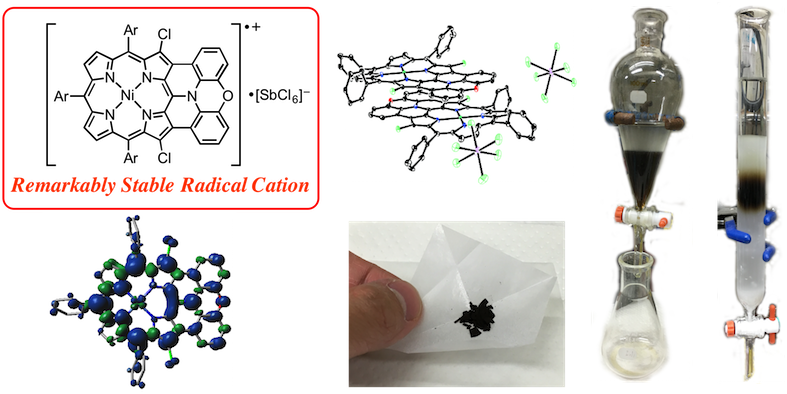
9. N. Fukui, W. Cha, D. Shimizu, J. Oh, K. Furukawa, H. Yorimitsu, D. Kim, A. Osuka
“Highly planar diarylamine-fused porphyrins and their remarkably stable radical cations”
Chem. Sci. 2017, 8, 189-199. [DOI: 10.1039/C6SC02721K] (Open access)
-
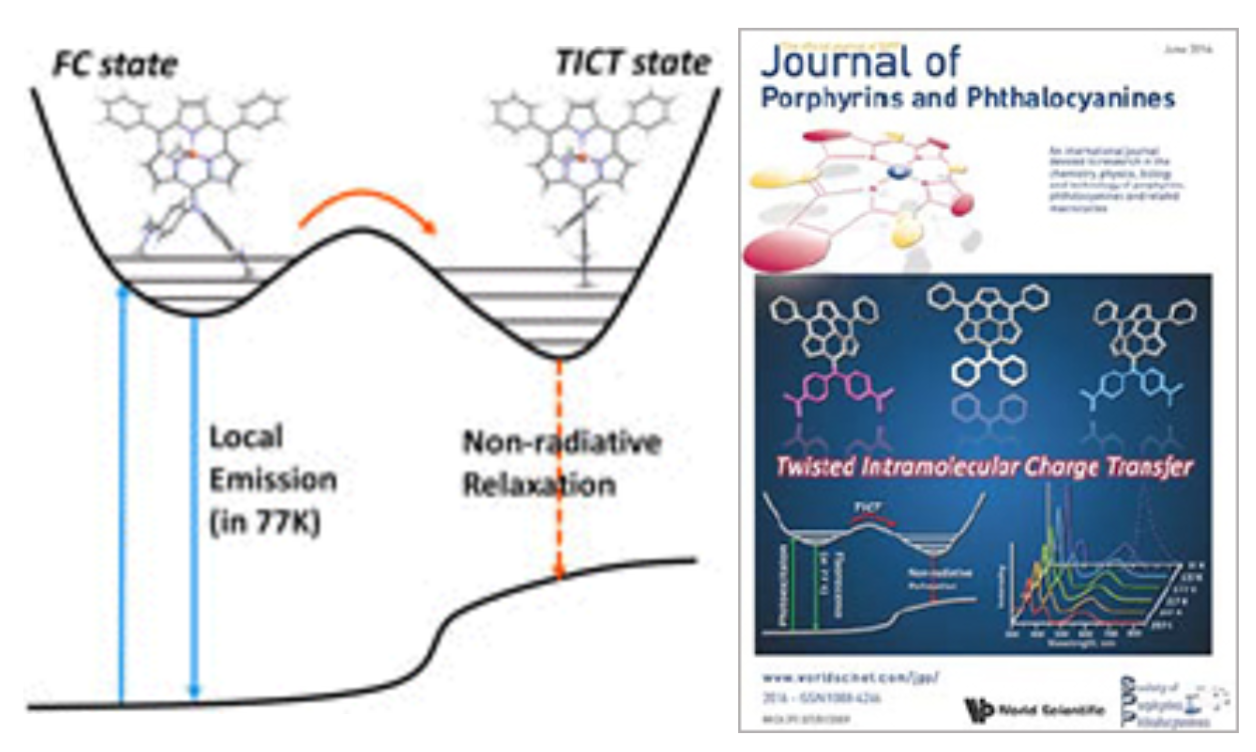
8. S.-K. Lee, J. O. Kim, D. Shimizu, A. Osuka, D. Kim
“Effect of bulky meso-substituents on photoinduced twisted intramolecular charge transfer processes in meso-diarylamino subporphyrins”
J. Porphyrins Phthalocyanines 2016, 20, 663-669. [DOI: 10.1142/S1088424616500723]
-
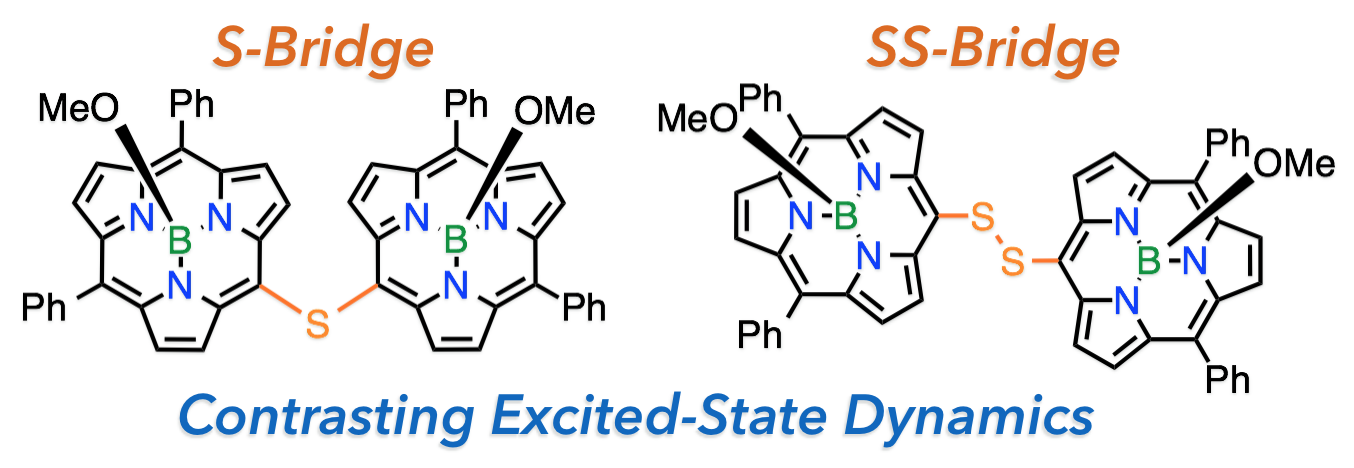
7. G. Copley, D. Shimizu, J. Oh, J. Sung, K. Furukawa, D. Kim, A Osuka
“meso-to-meso Sulfide- and Disulfide-Bridged Subporphyrin Dimers”
Eur. J. Org. Chem. 2016, 1977-1981. [DOI: 10.1002/ejoc.201600285]
-
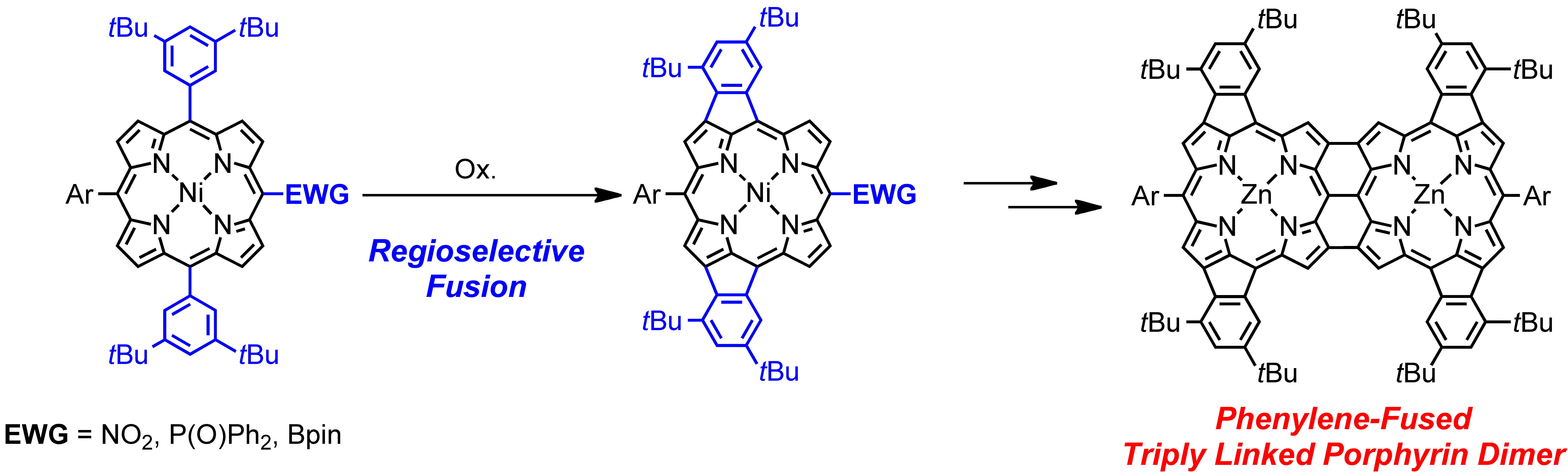
6. N. Fukui, S.-K. Lee, K. Kato, D. Shimizu, T. Tanaka, S. Lee, H. Yorimitsu, D. Kim, A. Osuka
“Regioselective phenylene-fusion reactions of Ni(II)-porphyrins controlled by an electron-withdrawing meso-substituent”
Chem. Sci. 2016, 7, 4059-4066. [DOI: 10.1039/C5SC04748J] (Open access)
-
5. G. Copley, J. Oh, K. Yoshida, D. Shimizu, D. Kim, A. Osuka
“Intramolecular electron transfer reactions in meso-(4-nitrophenyl)-substituted subporphyrins”
Chem. Commun. 2016, 52, 1424-1427. [DOI: 10.1039/c5cc09005a]

-
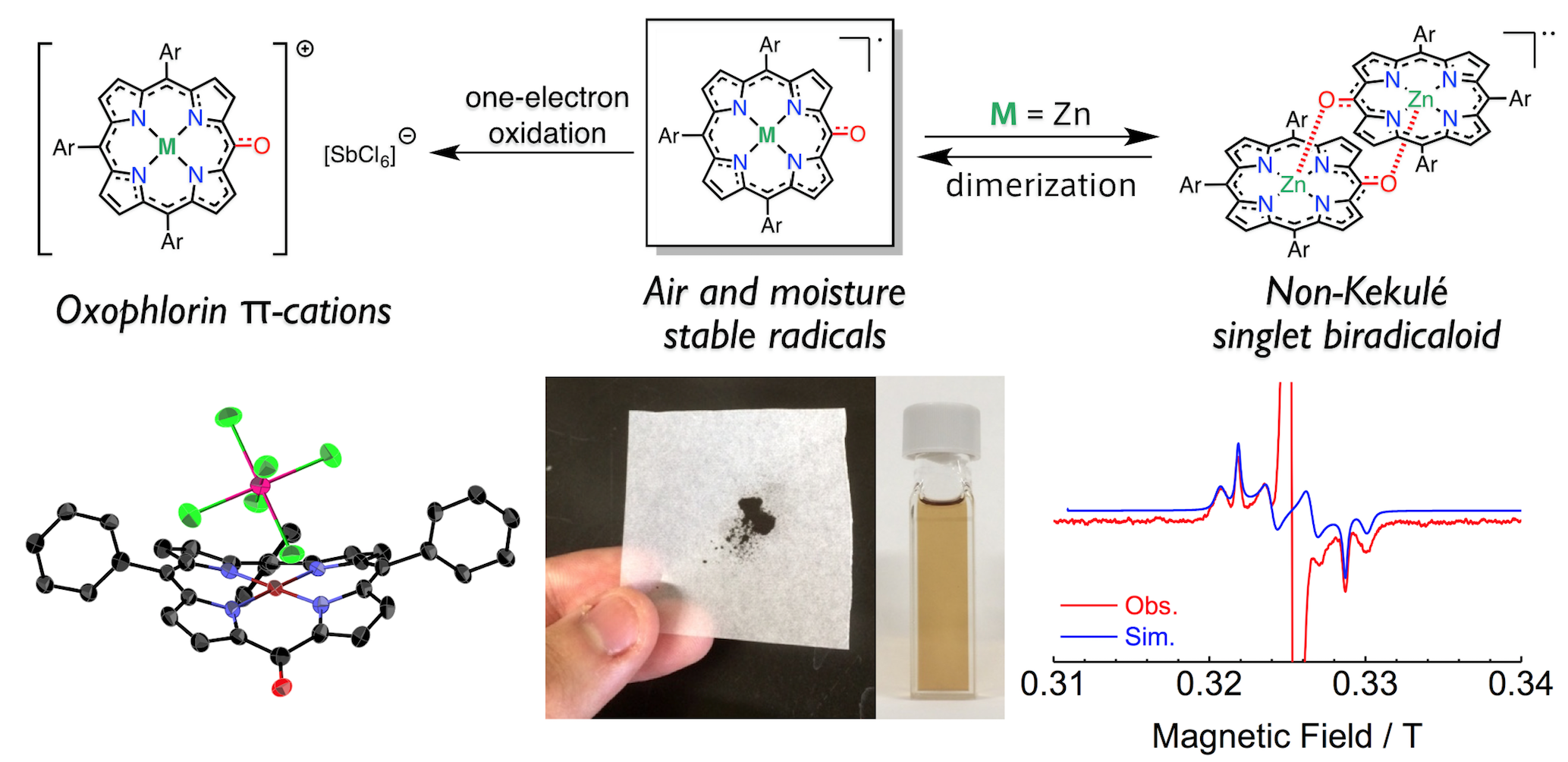
4. D. Shimizu, J. Oh, K. Furukawa, D. Kim, A. Osuka
“Triarylporphyrin meso-Oxy Radicals: Remarkable Chemical Stabilities and Oxidation to Oxophlorin π-Cations”
J. Am. Chem. Soc. 2015, 137, 15584-15594. [DOI: 10.1021/jacs.5b11223]
-
3. D. Shimizu, J. Oh, K. Furukawa, D. Kim, A. Osuka
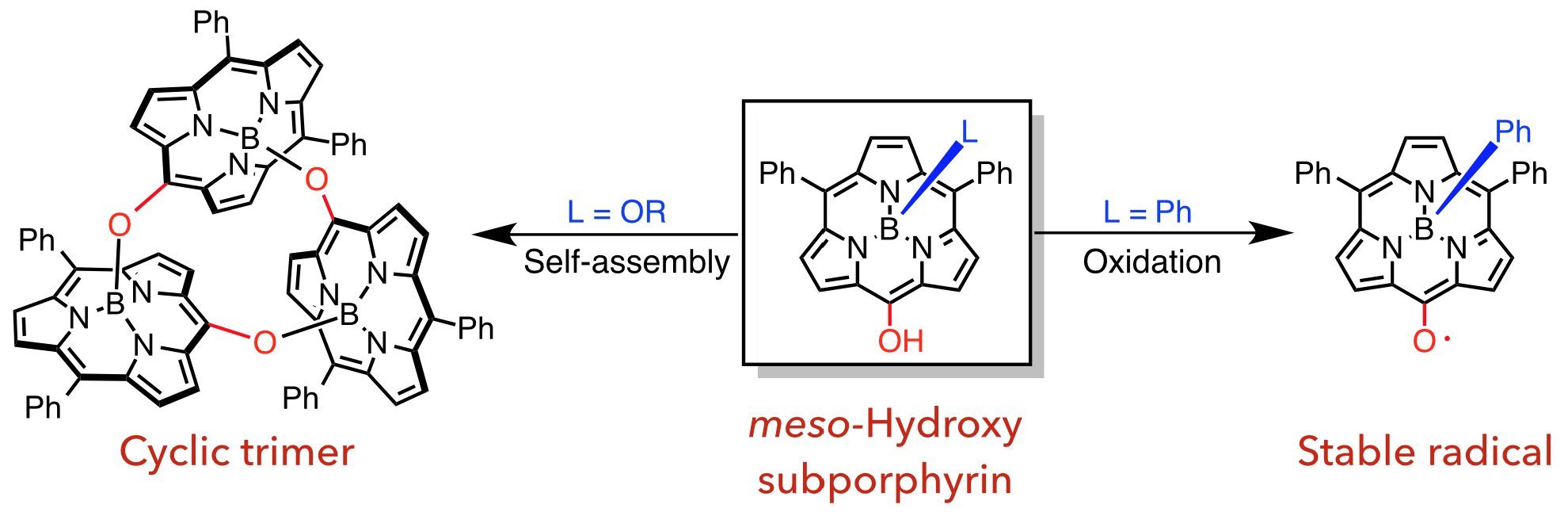
“meso-Hydroxysubporphyrins: a Cyclic Trimeric Assembly and a Stable meso-Oxy Radical”
Angew. Chem. Int. Ed. 2015, 54, 6613-6617. [DOI: 10.1002/anie.201501592]
-
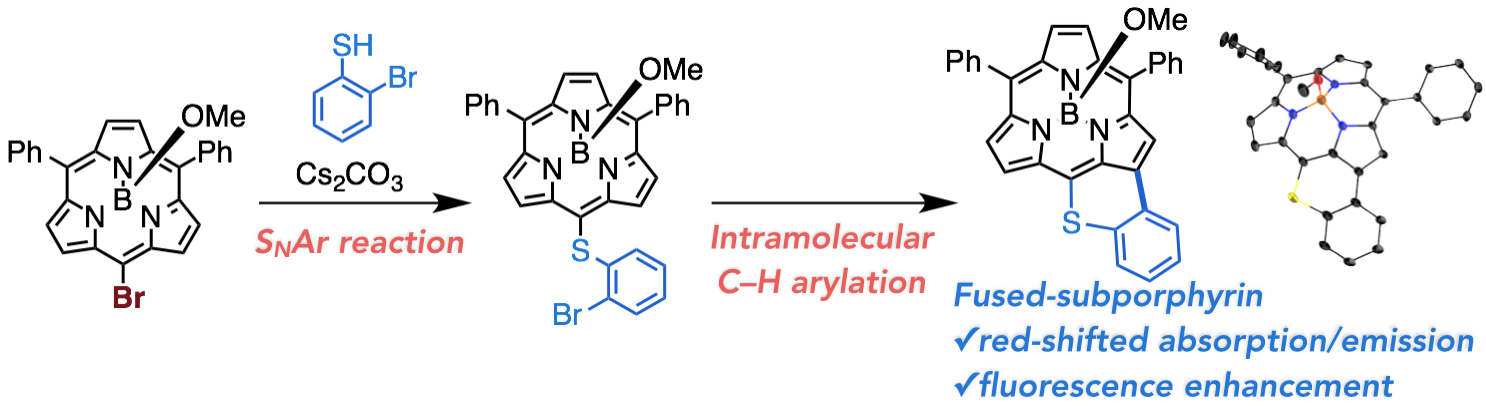
2. D. Shimizu, H. Mori, M. Kitano, W.-Y. Cha, J. Oh, T. Tanaka, D. Kim, A. Osuka
“Nucleophilic Aromatic Substitution Reactions of meso-Bromosubporphyrin: Synthesis of a Thiopyrane-Fused Subporphyrin””
Chem. Eur. J. 2014, 20, 16194-16202. [DOI: 10.1002/chem.201405110]
-
1. M. Kitano, D. Shimizu, T. Tanaka, H. Yorimitsu, A. Osuka
“Synthesis of meso-heteroatom-substituted subporphyrins”
J. Porphyrins Phthalocyanines 2014, 18, 659-665. [DOI: 10.1142/s1088424614500394]

総説・記事
-
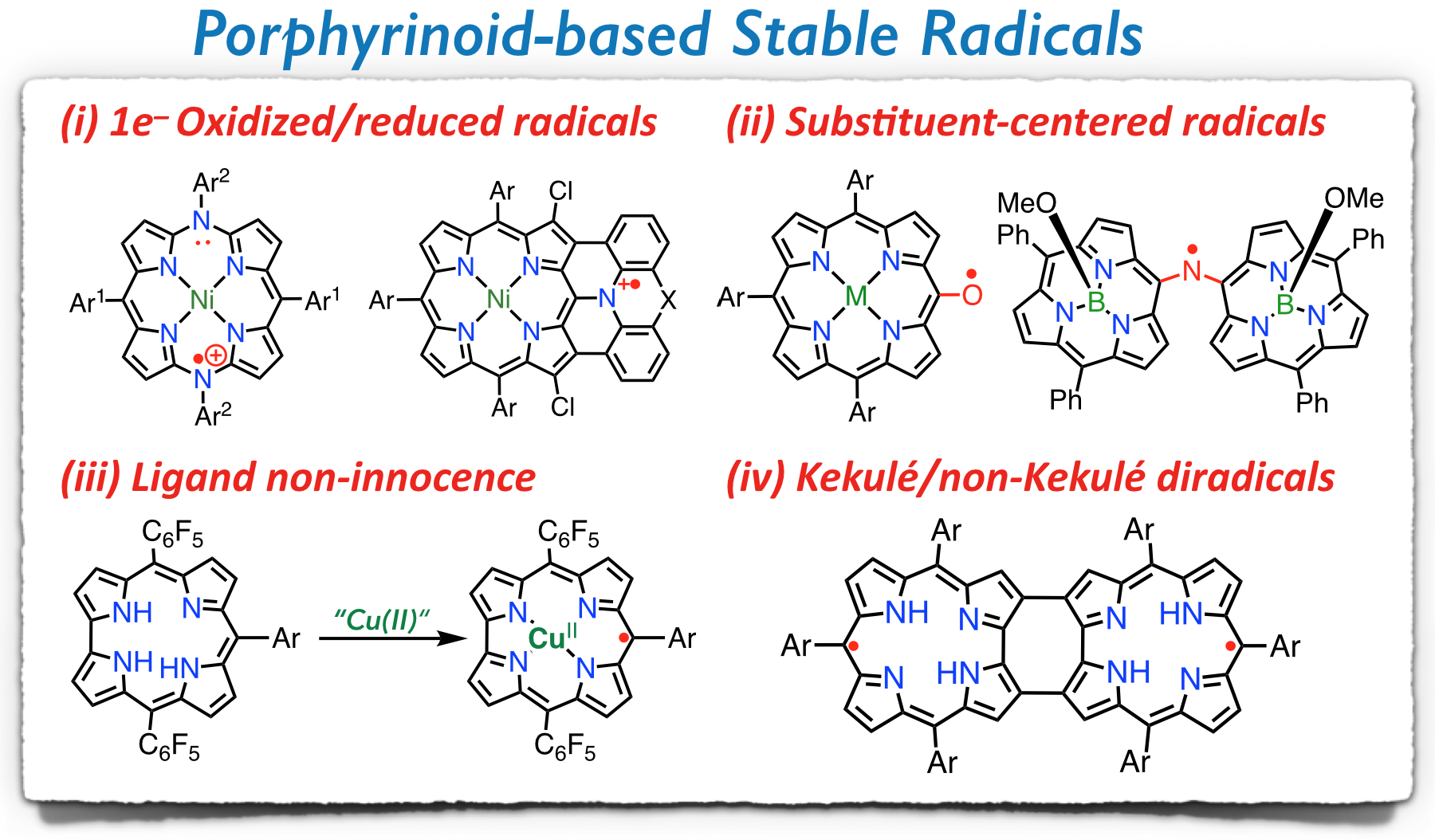
1. D. Shimizu and A. Osuka
“Porphyrinoids as a platform of stable radicals”
Chem. Sci. 2018, 9, 1408−1423. [DOI: 10.1039/C7SC05210C]
依頼・招待講演
-
14. Daiki Shimizu
“Taming exchange interaction in π-conjugation systems for functionalities”
The 2025 International Chemical Congress of Pacific Basin Societies (PACIFICHEM 2025)
Honolulu, Hawaii, 2025-12-15/20
-
13. Daiki Shimizu
“Exchange-Interaction-based Design of Functional π-Conjugation Systems”
Seminar at Hunan Normal University
Changsha, China, 2025-10-14
-
12. Daiki Shimizu
“安定ラジカル間にはたらく弱い交換相互作用の使いかた”
有機化学学生ウェビナー
オンライン, 2025-09-26
-
11. Daiki Shimizu
“Optically Functional Material Design based on the Weak Exchange Interaction in Multi-radical Systems”
Workshop on Spin-Related Optical Probes (Presymposium of the 15th International Conference on Optical Probes of Organic and Hybrid Semiconductors, OP2025)
Fukuoka, Japan, 2025-09-21/26
-
10. 清水 大貴
“ジラジカルの交換相互作用制御に基づく機能性有機分子系の創製と錯体への展開”
錯体化学会 第 75 回討論会
長崎大学 文教キャンパス, 2025-09-15/17
-
9. Daiki Shimizu
“Designing Functional π-Conjugated Systems based on Exchange Interaction”
The 10th Heron Island Conference on Reactive Intermediates and Unusual Molecules
Heron Island, Australia, 2025-07-06/12
-
8. Daiki Shimizu
“Optically Distinguishable Electronic Spin-isomers of a Stable Organic Diradical”
NanoSynergetics monthly web seminar
Online, 2024-06-13
-
7. 清水 大貴
“有機ジラジカルが示す多様な光学特性”
2023 年 光化学若手の会
津名ハイツ, 淡路島, 2023-06-09/11
-
6. 清水 大貴
“ジラジカルを「電子スピン異性体」と捉えることはできるか”
第 7 回有機若手ワークショップ
京都大学吉田キャンパス, 2022-11-29
-
5. Daiki Shimizu
“Spin-state dependent optical behavior of a stable Blatter radical dimer with through-space interaction”
The 9th International Kyoto Symposium on Organic Chemistry
(pre-Tateshina Conference/The 13th JGP Chem&ChemEn Workshop)
Kyoto, Japan, 2022-11-09
-
4. 清水 大貴
“スピン依存的な光励起挙動を示す開殻電子系の創出”
構造有機 Mirai セミナー
定山渓ビューホテル
札幌, 2022-06-24/25
-
3. 清水 大貴
“電荷やスピンを制御した新しいπ電子系の創製を目指して”
GTR Chemistry Workshop 2021
名古屋大学卓越大学院プログラム トランスフォーマティブ化学生命融合研究大学院プログラム
オンライン, 2021-11-29
-
2. 清水 大貴
“電荷や不対電子の導入に基づく新規な共役系の創製”
第15回物性科学領域横断研究会
オンライン, 2021-11-26
-
1. 清水 大貴
“安定ラジカルの基盤としてのポルフィリン類縁体”
2018 年度 高分子・ハイブリッド材料研究センター(PHyM)若手フォーラム
東北大学多元物質科学研究所
仙台, 2018-11-19



