Members
Daiki SHIMIZU

Assistant Professor
Department of Synthetic Chemistry and Biological Chemistry,
Graduate School of Engineering Kyoto University
A4-325, Kyoto University Katsura Campus,
Nishikyo-ku, Kyoto 615-8510, Japan
e-mail: dshimizu(at)sbchem.kyoto-u.ac.jp
Tel: +81-75-383-2746
Fax: +81-75-383-2741
Education
- 2013 B.S. Kyoto University
- 2016 M.S. Kyoto University
- 2019 Ph.D. Kyoto University (Advisor: Atsuhiro Osuka)
Professional Experiences
- 2019-04/present Assistant Professor Kyoto University
- 2017-08/2017-11 Visiting Scholar, Northwestern University (the Wasielewski Group)
- 2016-04/2019-03 JSPS Fellowship for Young Scientists (DC1)
Awards
- 2025-02 Kenichi Fukui Encouragement Award, Fukui Institute for Fundamental Chemistry, Kyoto University, Japan
- 2023-12 Mitsubishi Chemical Award in Synthetic Organic Chemistry, Society of Synthetic Organic Chemistry, Japan
- 2020-02 Inoue Research Award for Young Scientists, Inoue Foundation for Science
- 2018-12 Chemical Reviews Poster Prize, The 10th Singapore International Chemistry Conference (SICC-10)
- 2018-09 RSC Advances Award, The 29th Symposium on Physical Organic Chemistry
- 2017-08 Best Poster AwardThe 49th Summer School of Structural Organic Chemistry
- 2017-03 Student Presentation Award, Chemical Society of Japan
- 2016-10 Otsu Academy Award Fellow (No.107)
Original Papers
TOC:
Show
|
Hide
-
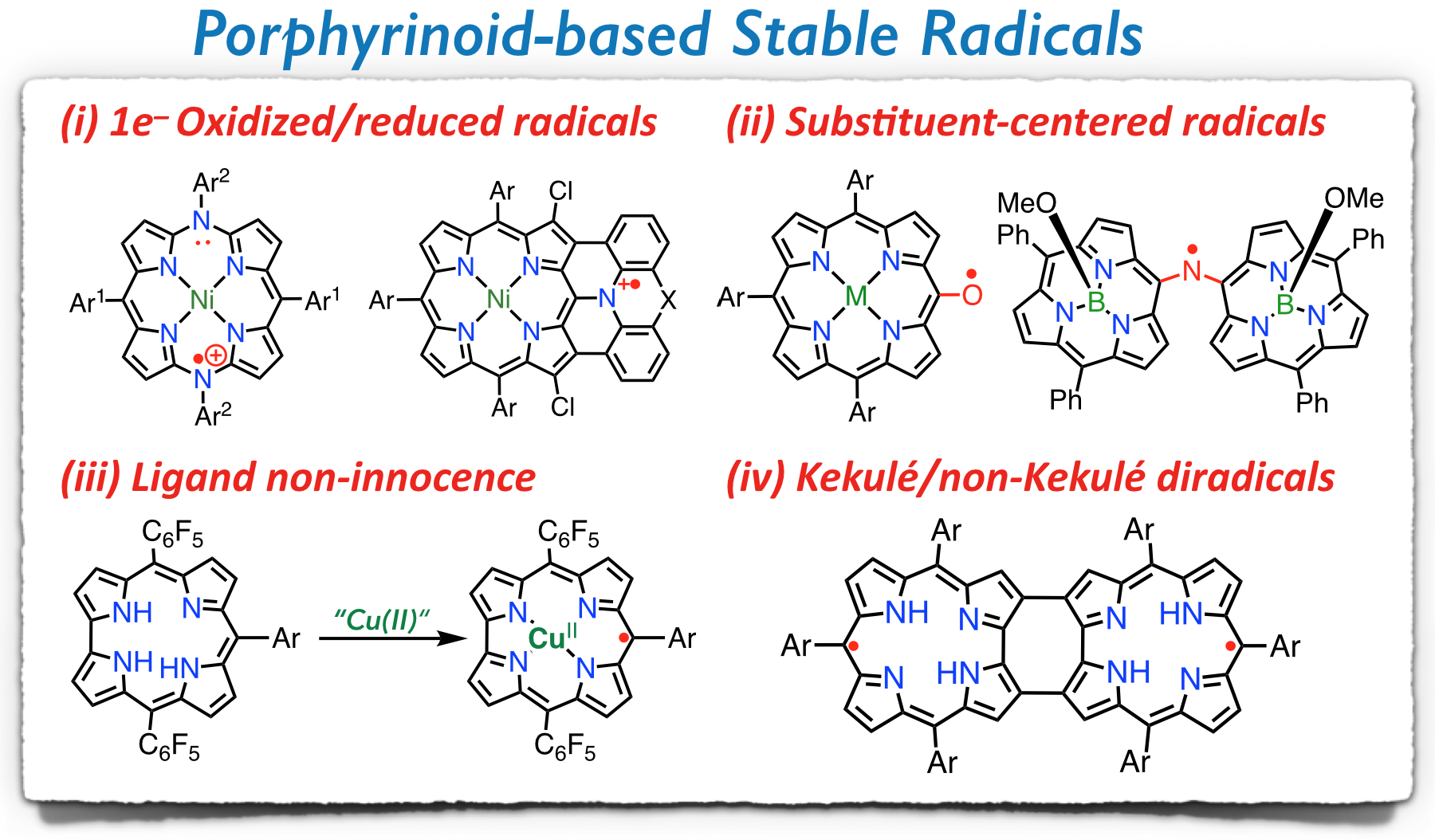
66. D. Shimizu, K. Matsuda
“Modulation of Koelsch Radical Stability and Aromaticity through Non-Hexagonal Ring Fusion”
[DOI: ///]
-
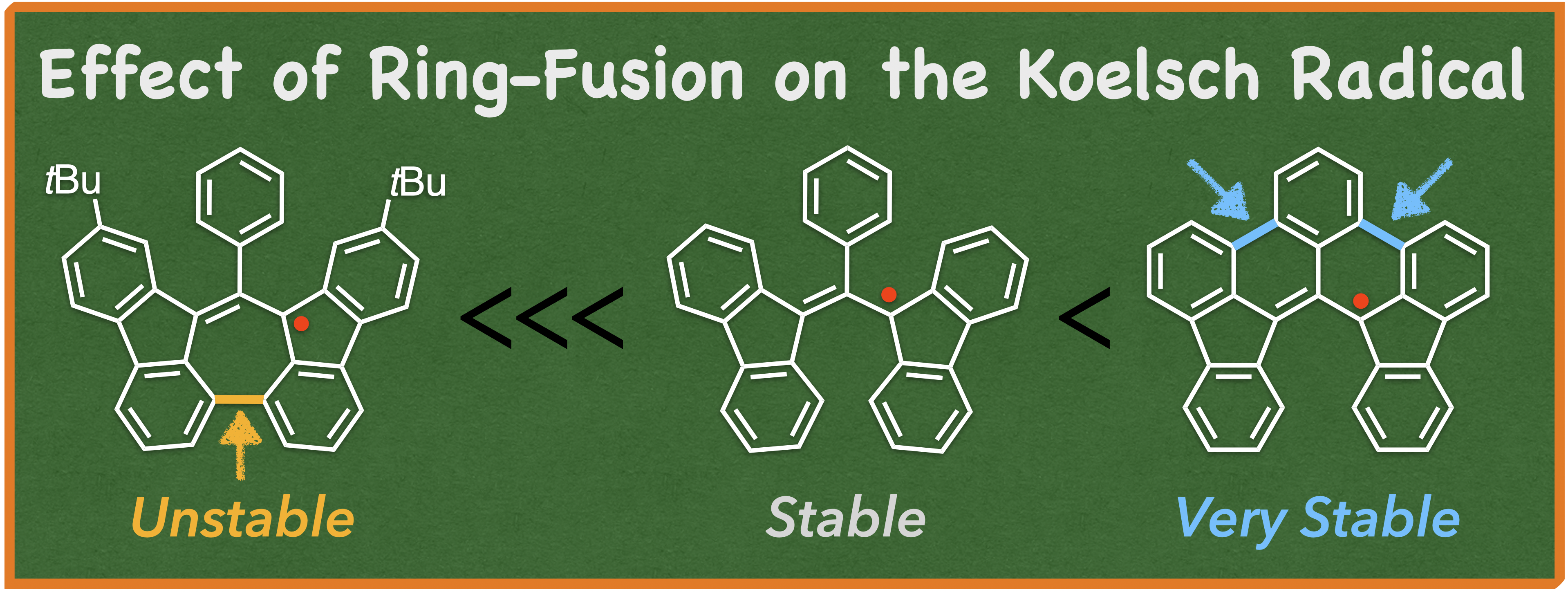
65. T. Shinozuka, D. Shimizu, K. Matsuda
“Computational Study on the Relationship between Charge Localization and Second Hyperpolarizability in Mixed-Valence Systems”
Chem. Eur. J. 2025, 31, e202501019. [DOI: 10.1002/chem.202501019]
-
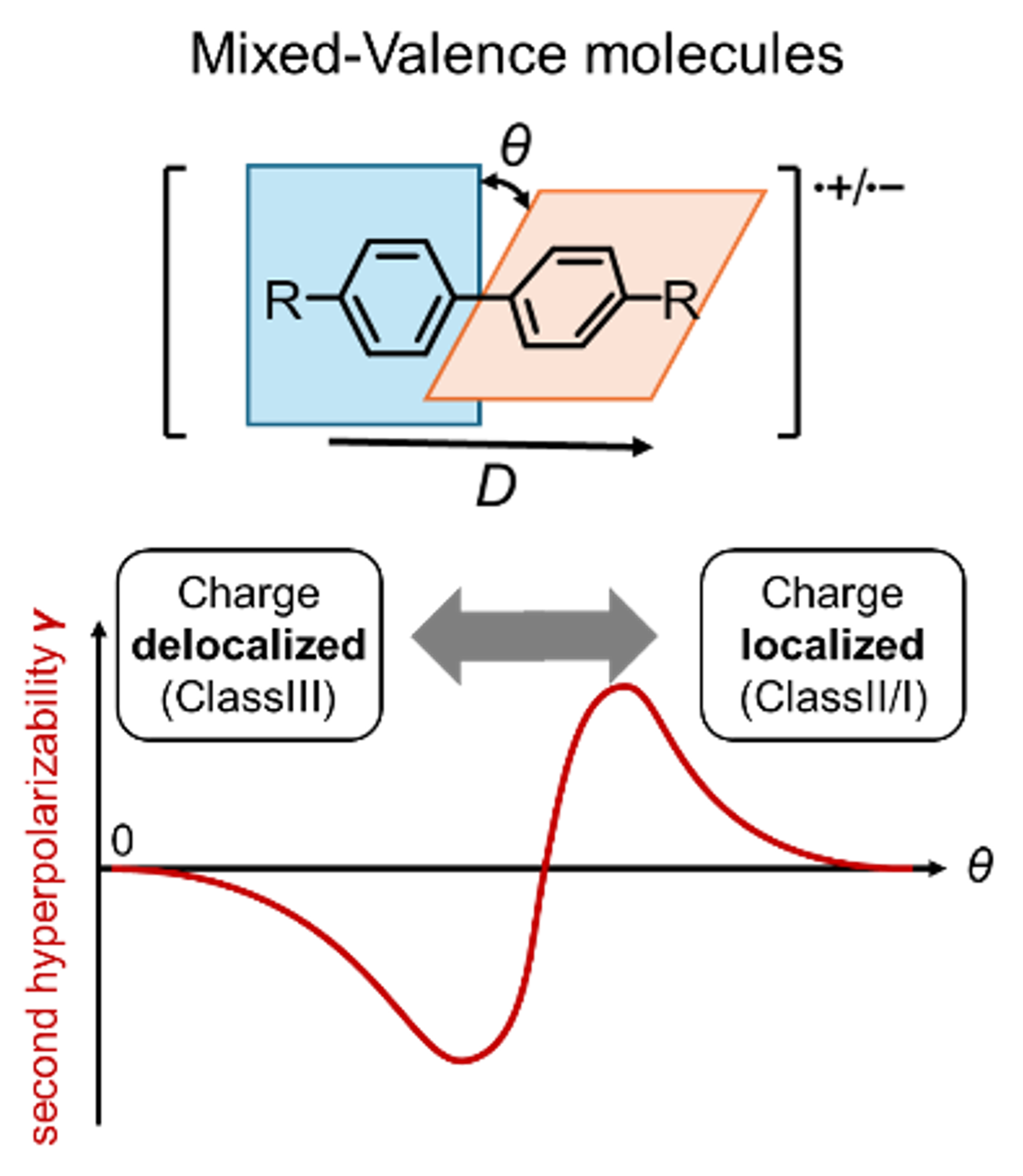
64. Y. Ono, Y. Goto, Y. Tani, K. Nakasuji, K. Sato, T. Takui, D. Shimizu, K. Matsuda, T. Kubo
“Benzo[cd]perylenyl: A π-Expanded Phenalenyl Radical with Enhanced Aggregation Enthalpy and Stability”
Asian J. Org. Chem. 2025, 14, e202500300. [DOI: 10.1002/ajoc.202500300]
-
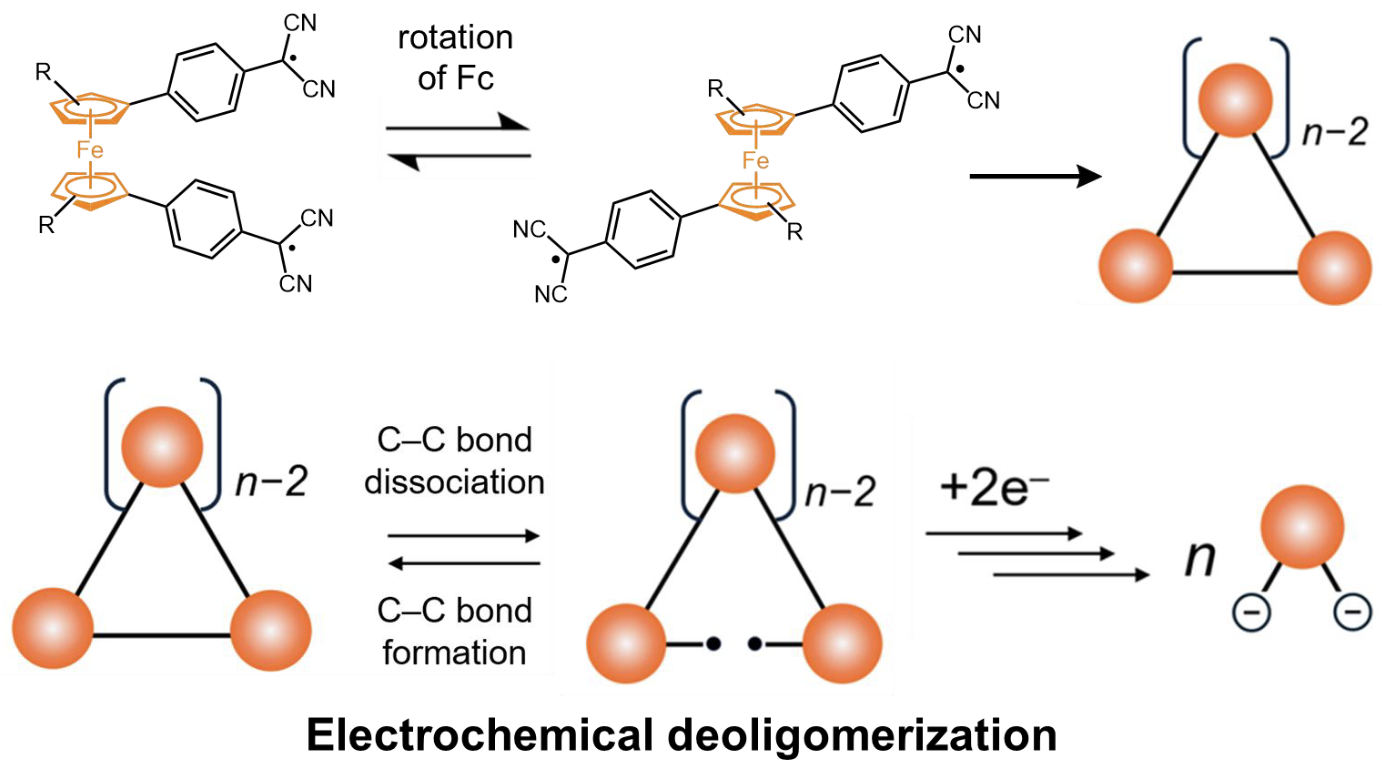
63. D. Sakamaki, K. Asano, D. Shimizu, H. Fujiwara
“Redox Control of Radical-Based Dynamic Covalent Chemistry: Macrocycle Formation and Electrochemical Deoligomerization of Bis(Dicyanomethyl Radical)-Substituted Ferrocene”
Asian J. Org. Chem. 2025, 14, e202500253. [DOI: 10.1002/ajoc.202500253]
-
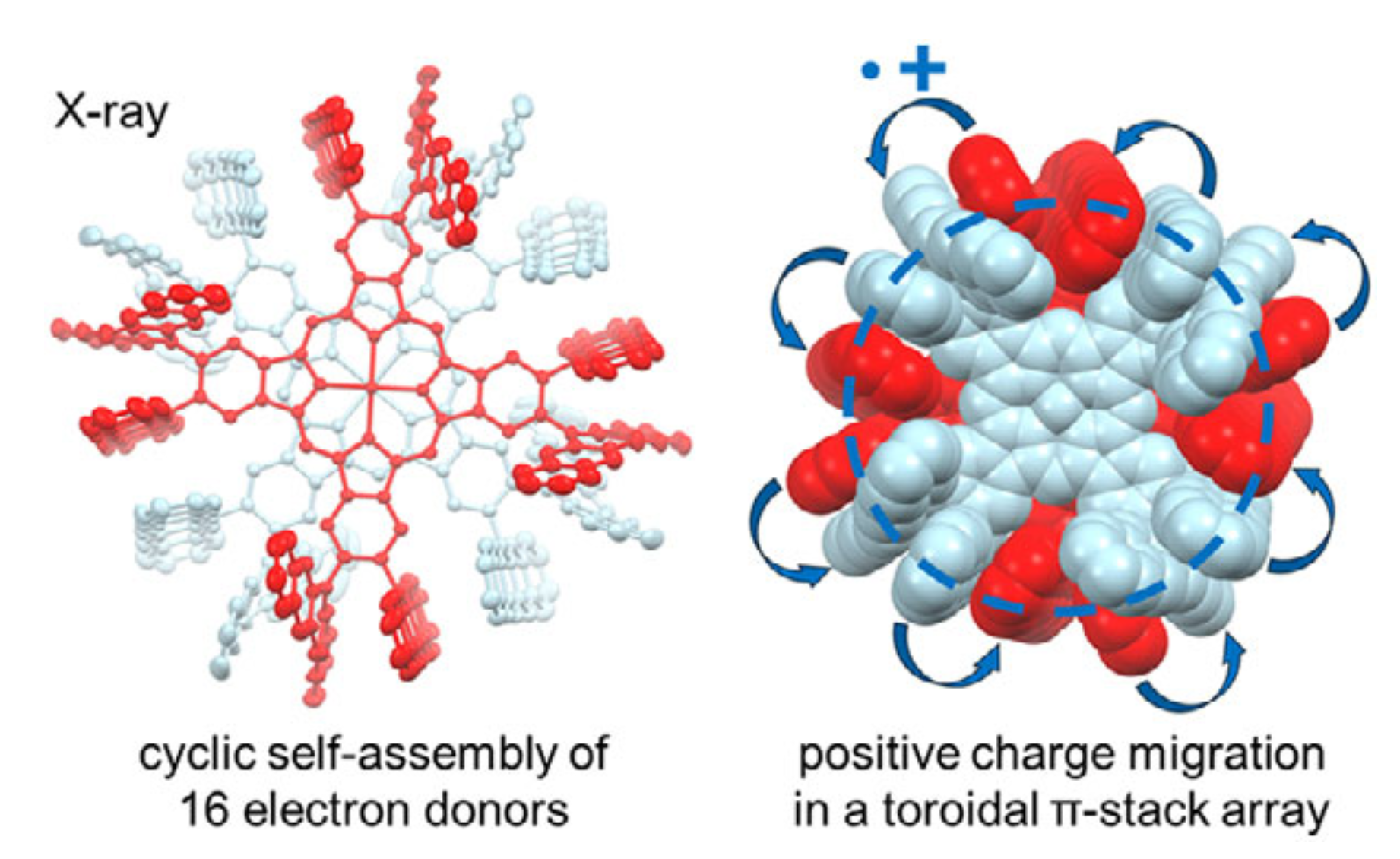
62. D. Sakamaki, K. Tsubono, M. Nakamura, D. Shimizu, Y. Matsui, H. Ikeda, K. Furukawa, H. Fujiwara
“Intermolecular Toroidal Conjugation in Cylindrically Aligned Sixteen π-Planes Formed by Self-dimerization of Octa-substituted Phthalocyanines”
Angew. Chem. Int. Ed. 2025, 64, e202504353. [DOI: 10.1002/anie.202504353]
-
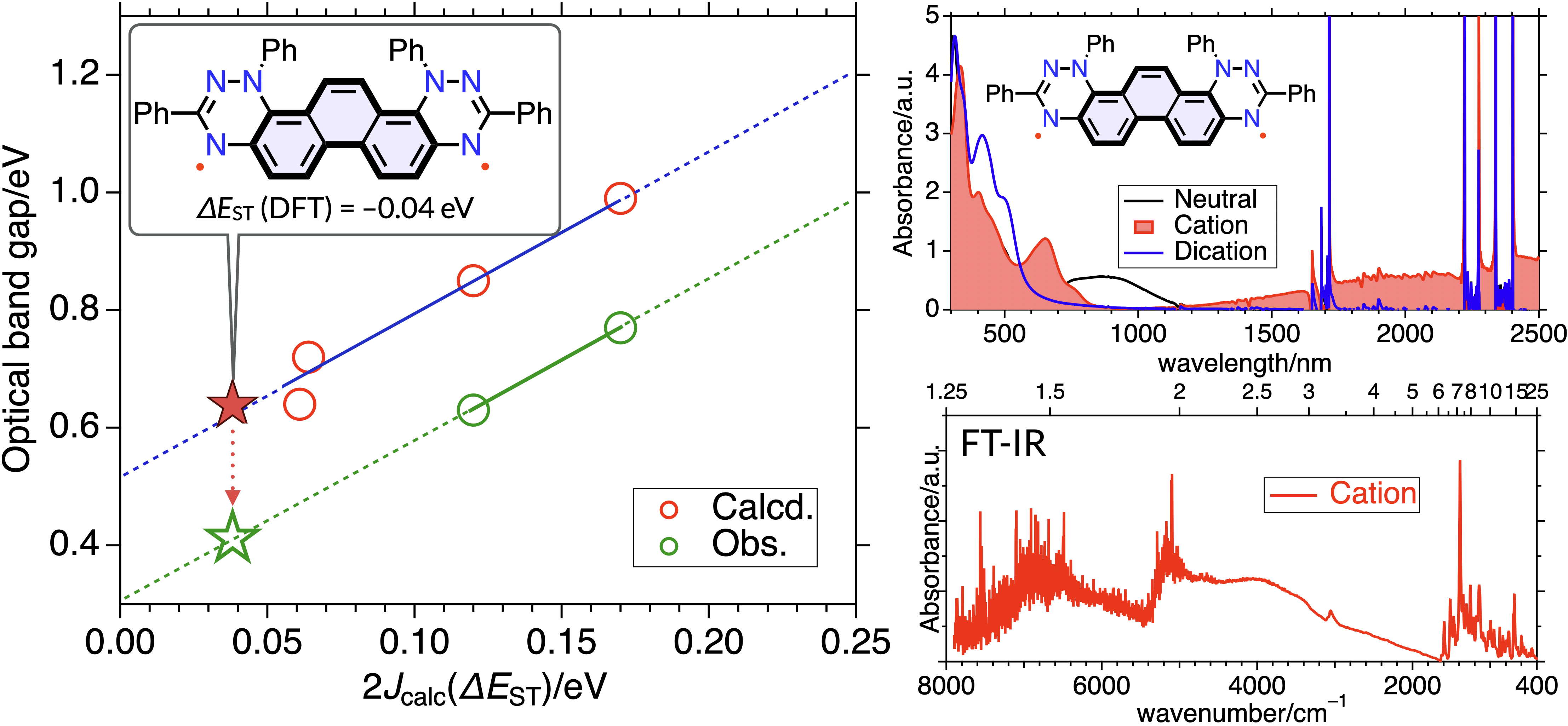
61. Y. Fujimoto, D. Shimizu, K. Matsuda
“Rational Design of a Metal-free Organic Radical Cation Exhibiting Mid-IR π-π* Transition based on the Exchange Interaction”
Manuscript submitted. [DOI: ///]
-
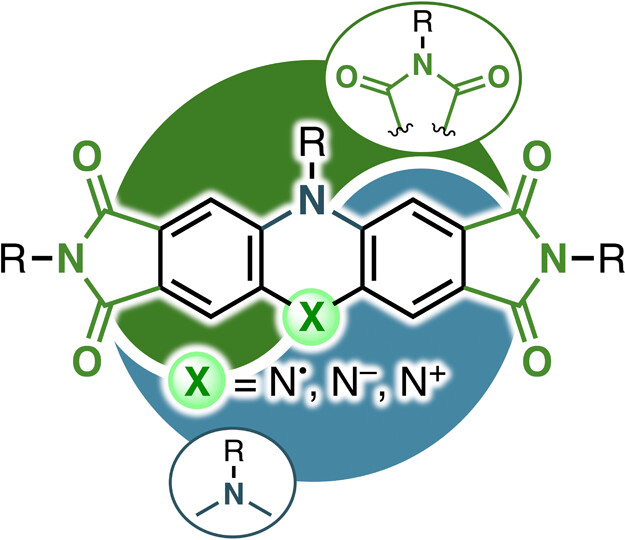
60. K. Tajima, C. Bucher, D. Shimizu, N. Fukui, H. Shinokubo
“Captodative Approach to Stable Nitrogen-Centered Radicals, Anions, and Cations Exhibiting Near-Infrared Electrochromism”
JACS Au 2025, 5, 1421-1428. [DOI: 10.1021/jacsau.5c00037]
-
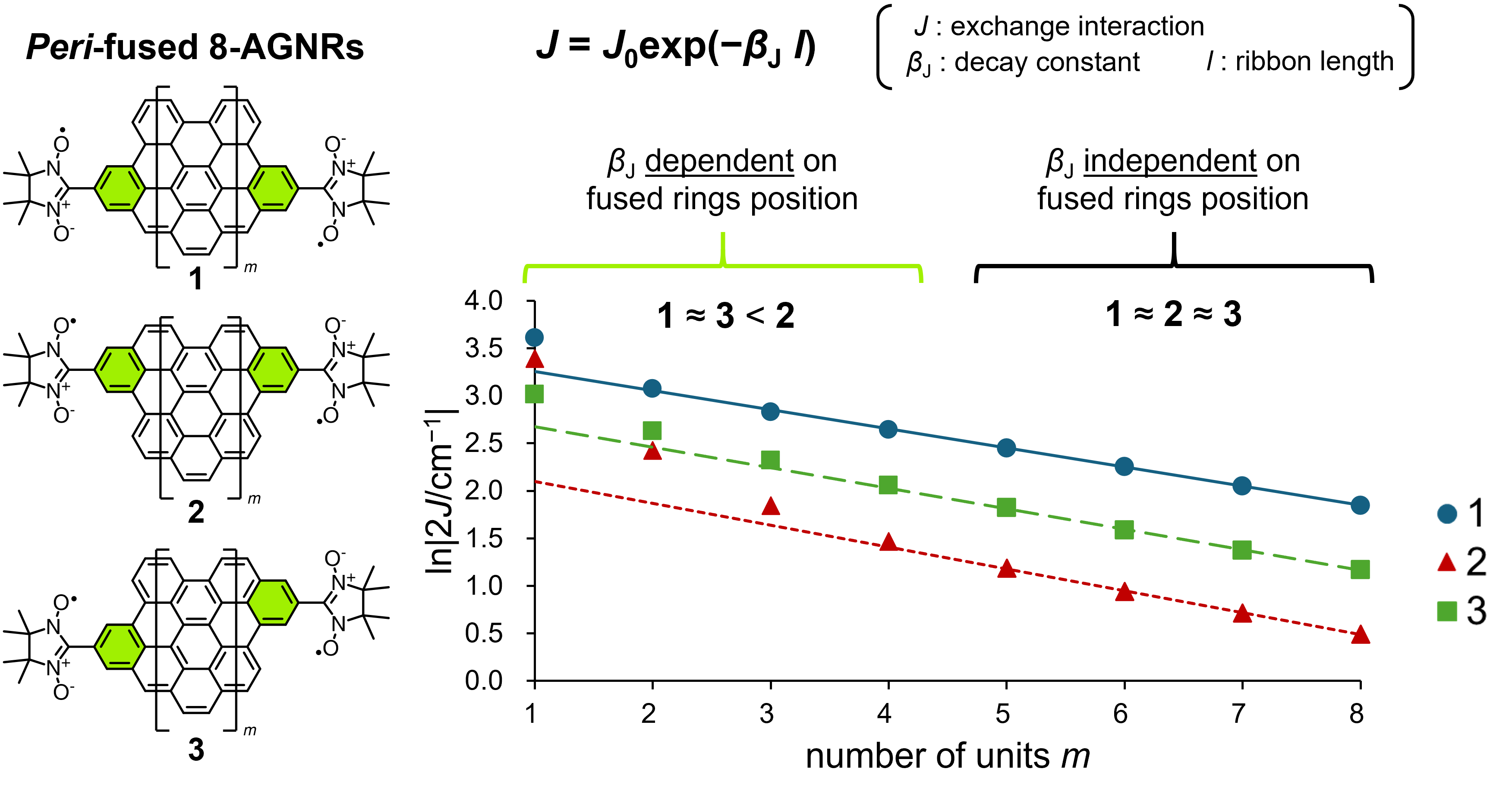
59. T. Shinozuka, D. Shimizu, K. Matsuda
“The effect of peri-fusion position on the single molecular conductivity of AGNRs theoretically evaluated by decay constant of exchange interaction”
Chem. Lett. 2024, 53, upae225. [DOI: 10.1093/chemle/upae225]
-
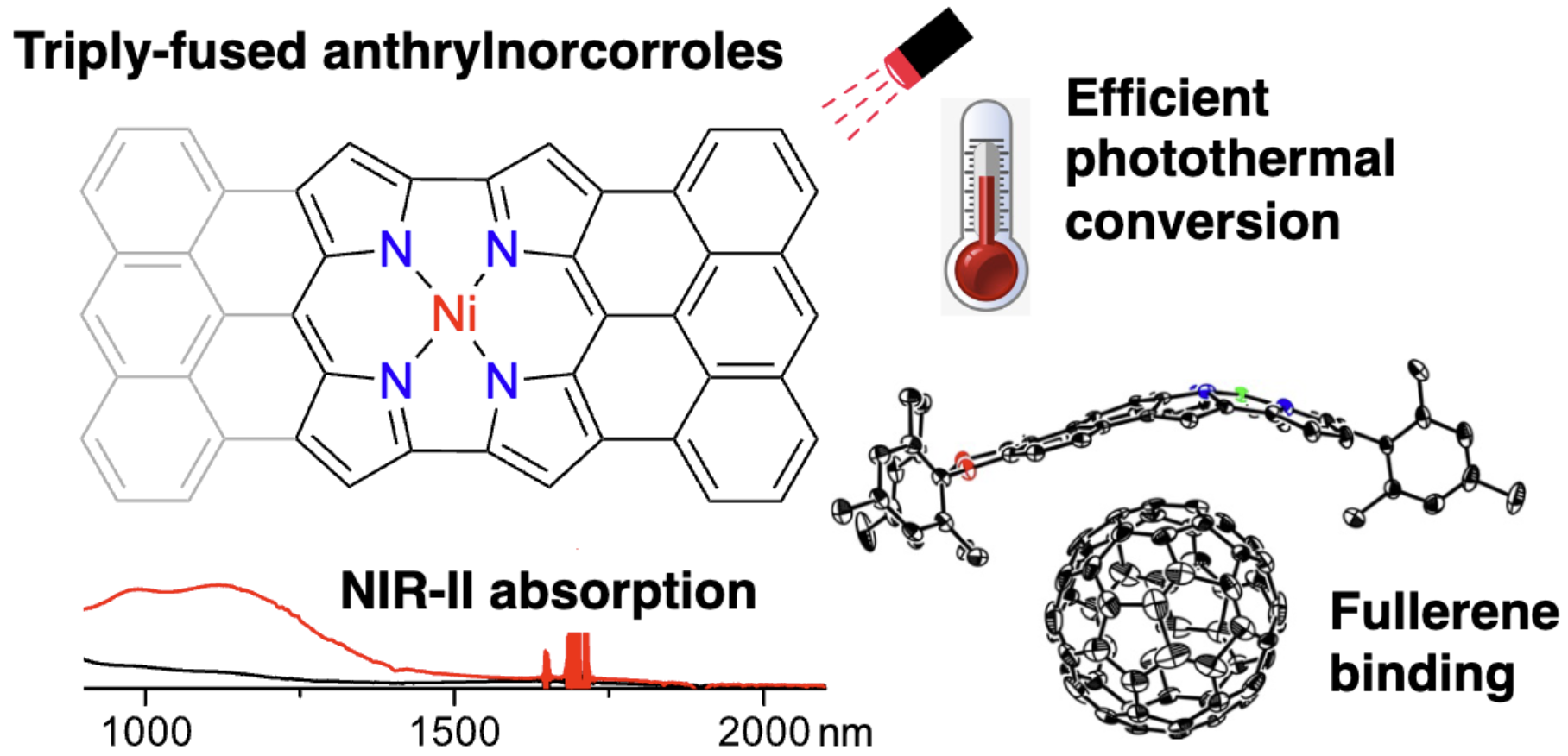
58. K. Wang, A. Ghosh, D. Shimizu, H. Takano, M. Ishida, R. Kishi, H. Shinokubo
“Bowl-Shaped Anthracene-Fused Antiaromatic Ni(II) Norcorrole: Synthesis, Structure, Assembly with C60, and Photothermal Conversion”
Angew. Chem. Int. Ed. 2025, 64, e202419289. [DOI: 10.1002/anie.202419289] (Open access)
*Press Release
-
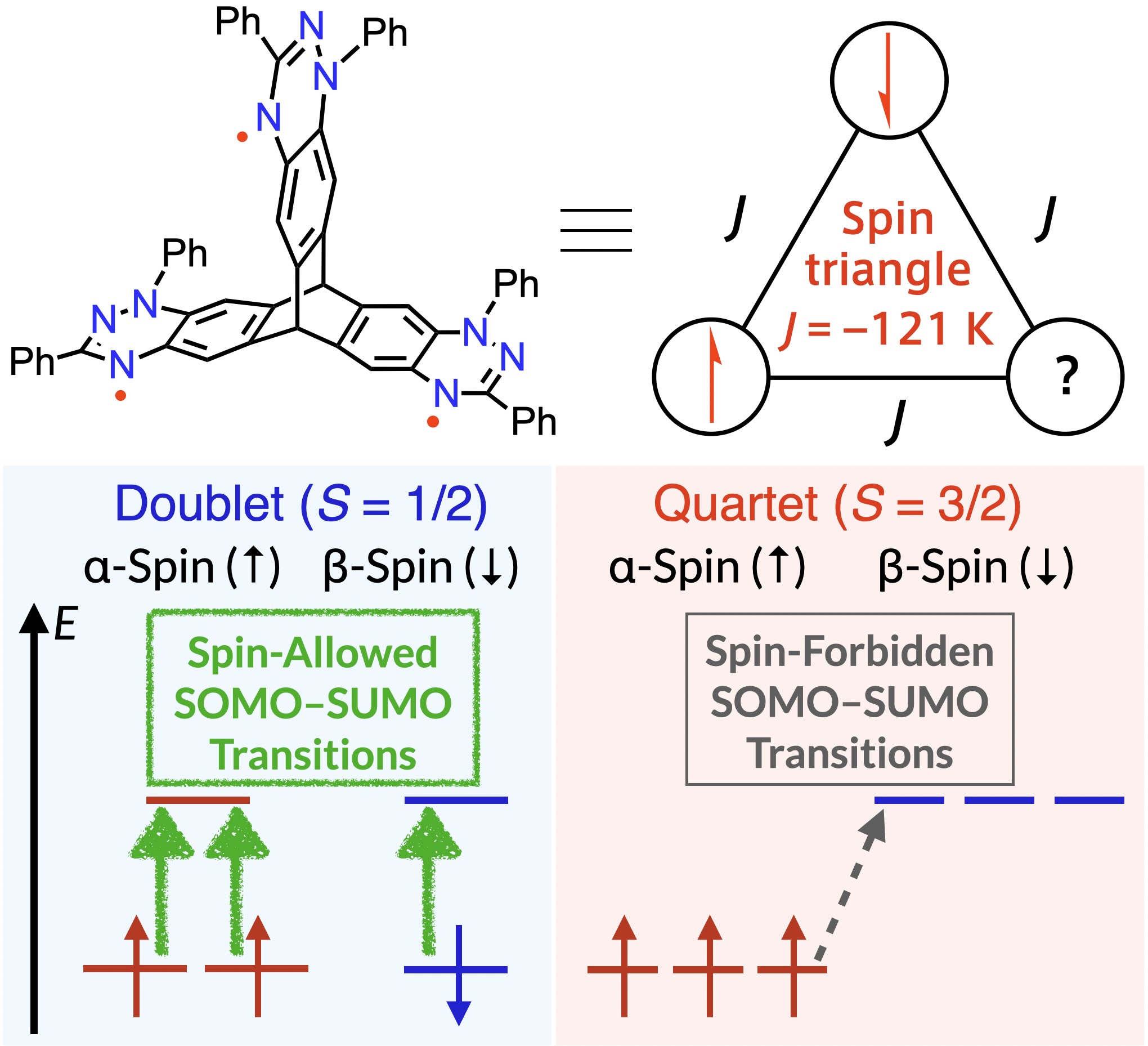
57. T. Aoki, H. Sotome, D. Shimizu, H. Miyasaka, K. Matsuda
“Propeller-Shaped Blatter-Based Triradicals: Distortion-Free Triangular Spin System and Spin-State-Dependent Photophysical Properties”
Angew .Chem. Int. Ed. 2025, 64, e202418655 (Hot Paper). [DOI: 10.1002/anie.202418655]
-
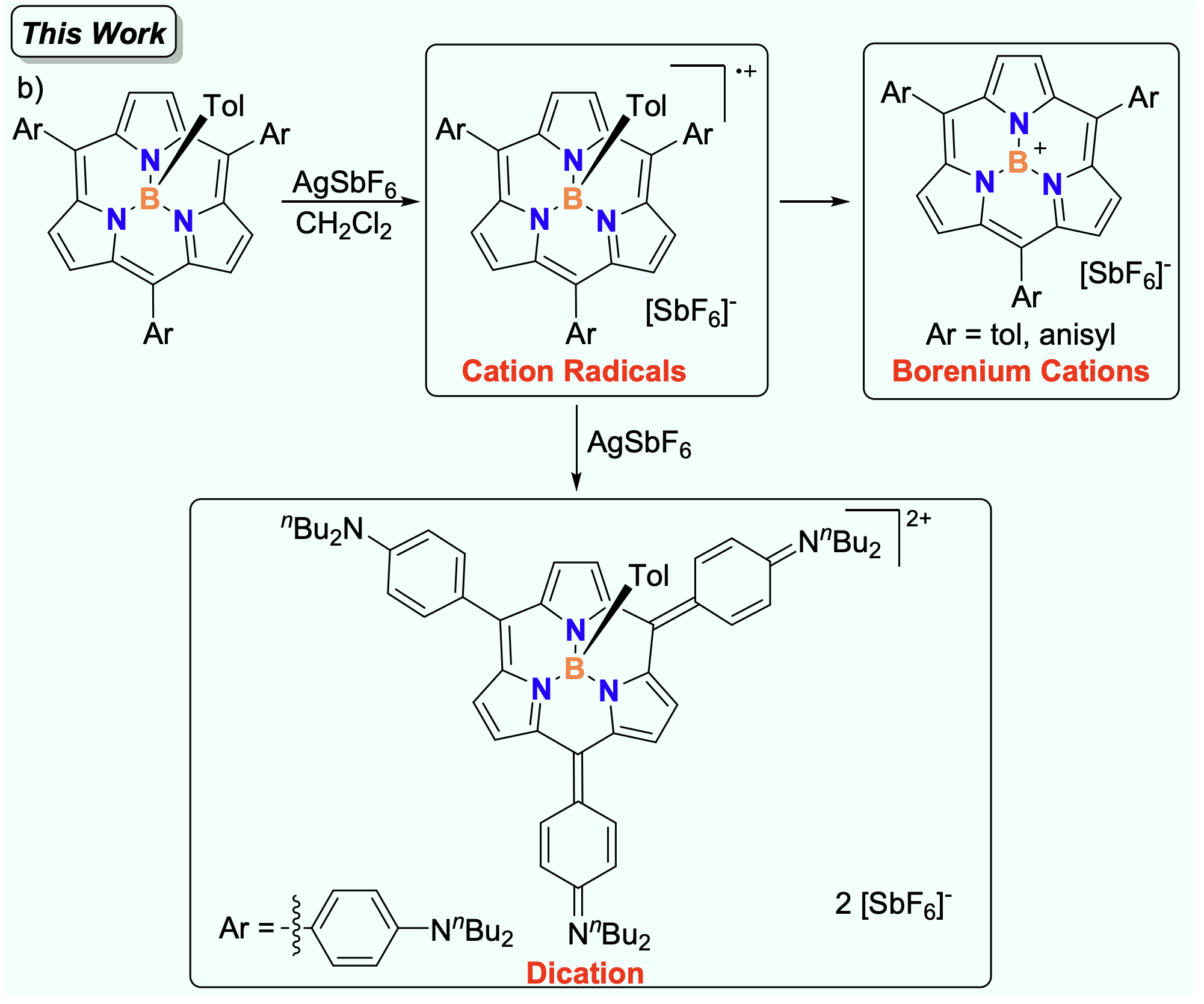
56. Z. Xie, X. Ji, X. Zeng, D. Shimizu, T. Tanaka, Y. Rao, M. Zhou, L. Xu, A. Osuka, J. Song
“Cation Radicals, Borenium Cations, and Dication from Oxidation of B-Tolyl BIII Subporphyrins”
Org. Chem. Front. 2025, 12, 1565-1571. [DOI: 10.1039/D4QO02291B]
-
55. T. Yamada. D. Shimizu, K. Matsuda
“Oxidation of Weakly Interacting Diradicals: an Approach for Strong and Tunable NIR-absorbing Dyes based on Small Chromophores”
J. Phys. Chem. Lett. 2024, 15, 9175-9182. [DOI: 10.1021/acs.jpclett.4c02212]
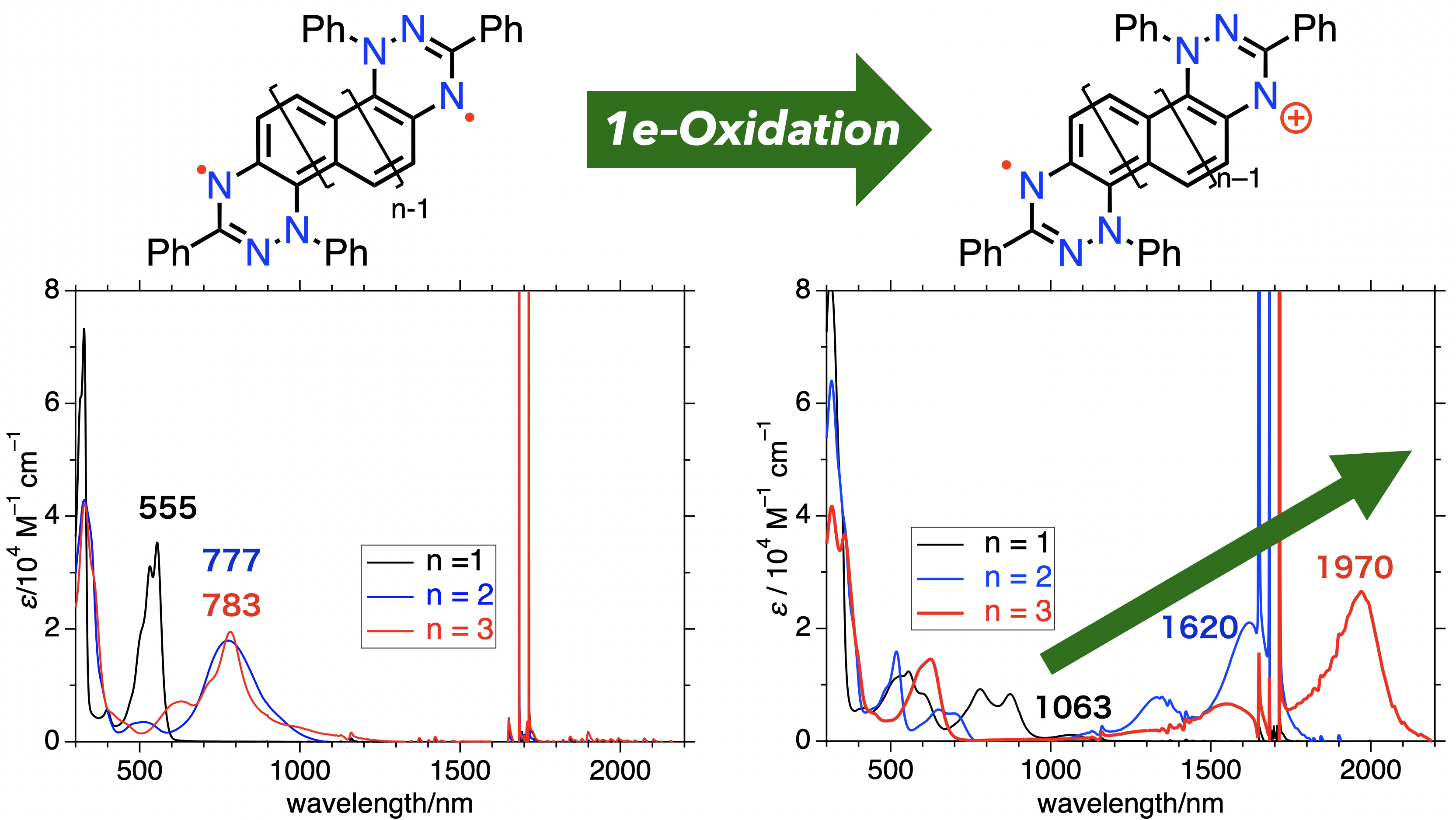
-
54. Y. Liu, T. Tanaka, D. Shimizu, Y. Rao, L. Xu, B. Yin, M. Zhou, J. Song, A. Osuka
“Fused Aromatic and Antiaromatic Smaragdyrin Dimers”
Angew. Chem. Int. Ed. 2024, 63, e202408478. [DOI: 10.1002/anie.202408478]
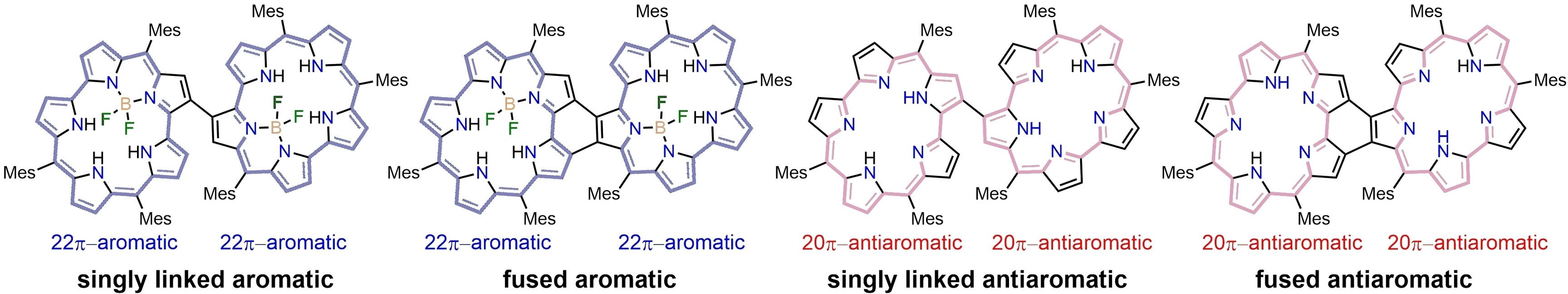
-
53. L. Wang, Z. Liao, P. Lin, Y. Jia, L. Liu, L. Xu, M. Zhou, B. Yin, Y. Rao, A. Nakai, T. Tanaka, D. Shimizu, A. Osuka, J. Song.
“Synthesis of NiII Porphyrin—NiII 5,15-Diazaporphyrin Hybrid Tapes“
Chem. Sci. 2024, 15, 10207-10213. [DOI: 10.1039/D4SC01450B] (Open access)
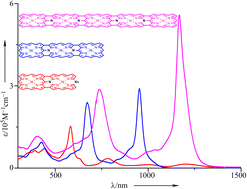
-
52. J. Chen, L. Liu, Y. Rao, L. Xu, M. Zhou, B. Yin, S. Shimizu, D. Shimizu, A. Osuka, J. Song
“[22]Pentaphyrins(2.0.1.1.0) Displaying N-Fusion, Pyrrole-Rearrangement, and Dimerization Reactions Upon Oxidation and Metalation”
Angew. Chem. Int. Ed. 2024, 63, e202407340. [DOI: 10.1002/anie.202407340]
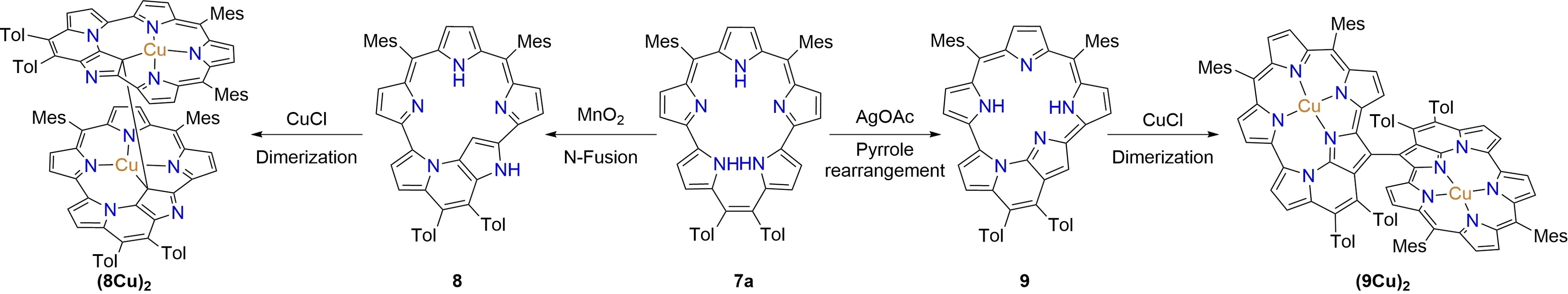
-
51. H. Hamamoto, D. Shimizu, K. Matsuda
“peri-Benzo-Diindenotetracenyl: Helically π-Extended Allyl Radical with Robust Stability”
Chem. Eur. J. 2024, 30, e202401353. [DOI: 10.1002/chem.202401353]
*Highlighted in Synfacts, 2024, 20, 1040.

-
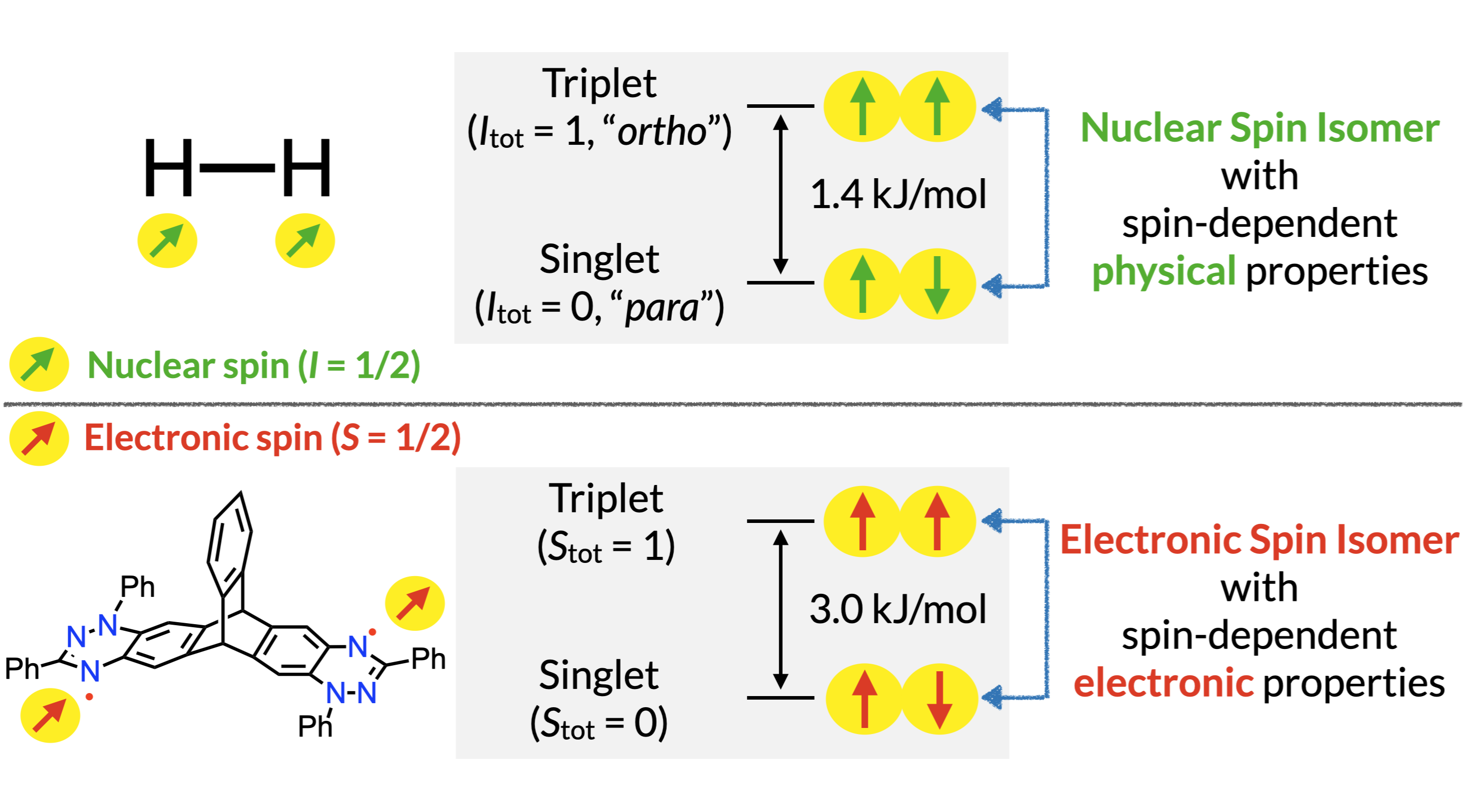
50. D. Shimizu, H. Sotome, H. Miyasaka, K. Matsuda
“Optically Distinguishable Electronic Spin-isomers of a Stable Organic Diradical”
ACS Cent. Sci. 2024, 10, 890-898. [DOI: 10.1021/acscentsci.4c00284] (Open access)
*Press Release [KyotoU] [OsakaU]
*Highlighted in Chem-Station
-
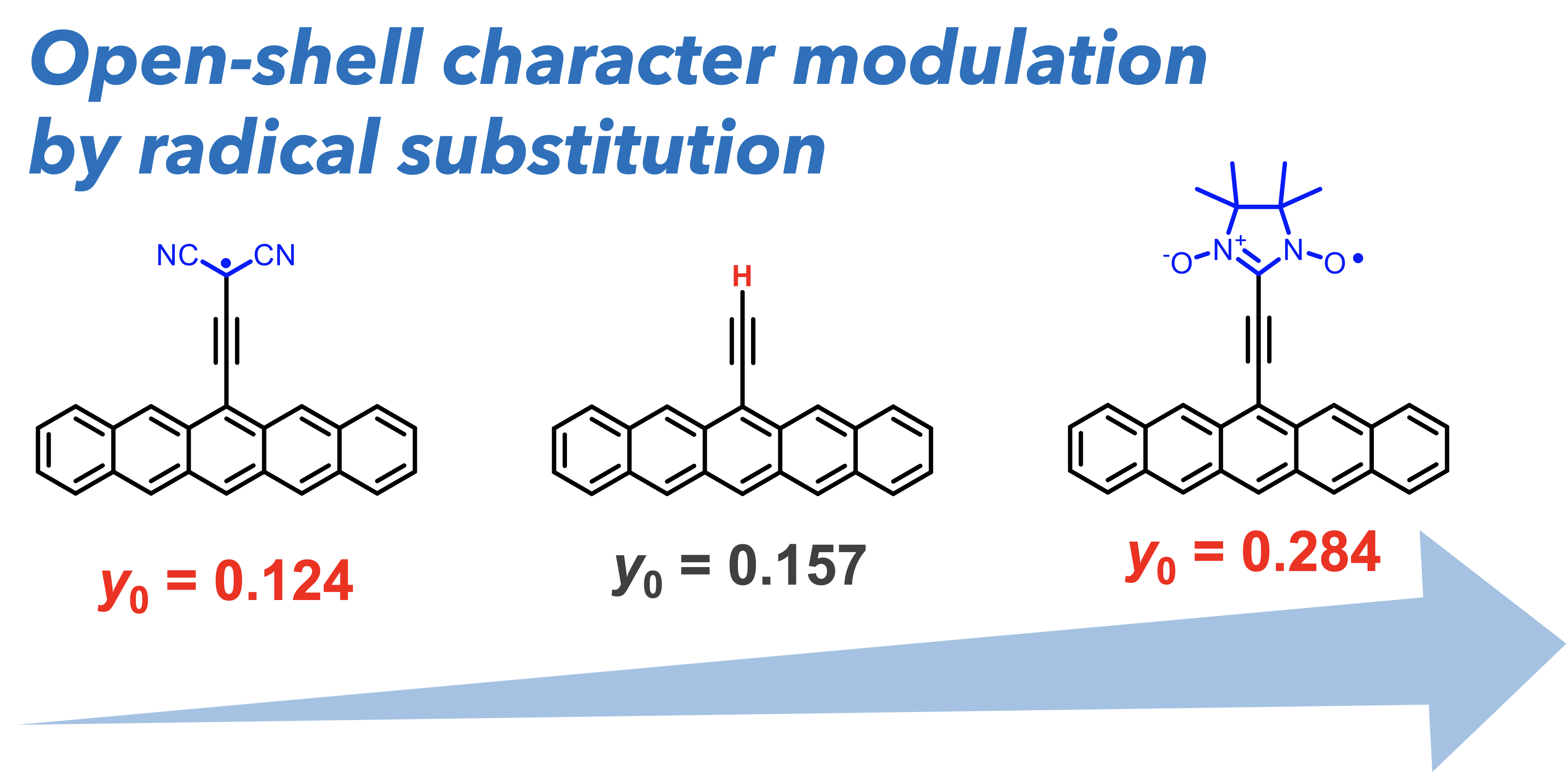
49. T. Shinozuka, D. Shimizu, K. Matsuda
“Theoretical investigation on the effect of radical substituents on the open-shell character of polycyclic aromatic hydrocarbon”
New J. Chem. 2024, 48, 8683-8689. [DOI: 10.1039/D4NJ00555D] (Open access)
-
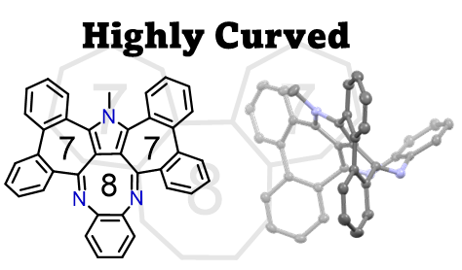
48. M. Hisada, D. Shimizu, K. Matsuda
“Synthesis and properties of doubly diphenylene-fused benzopyrrolo[1,4]diazocine with a [7-8-7] successive ring-fused structure”
Chem. Lett. 2024, 53, upae021. [DOI: 10.1093/chemle/upae021]
-
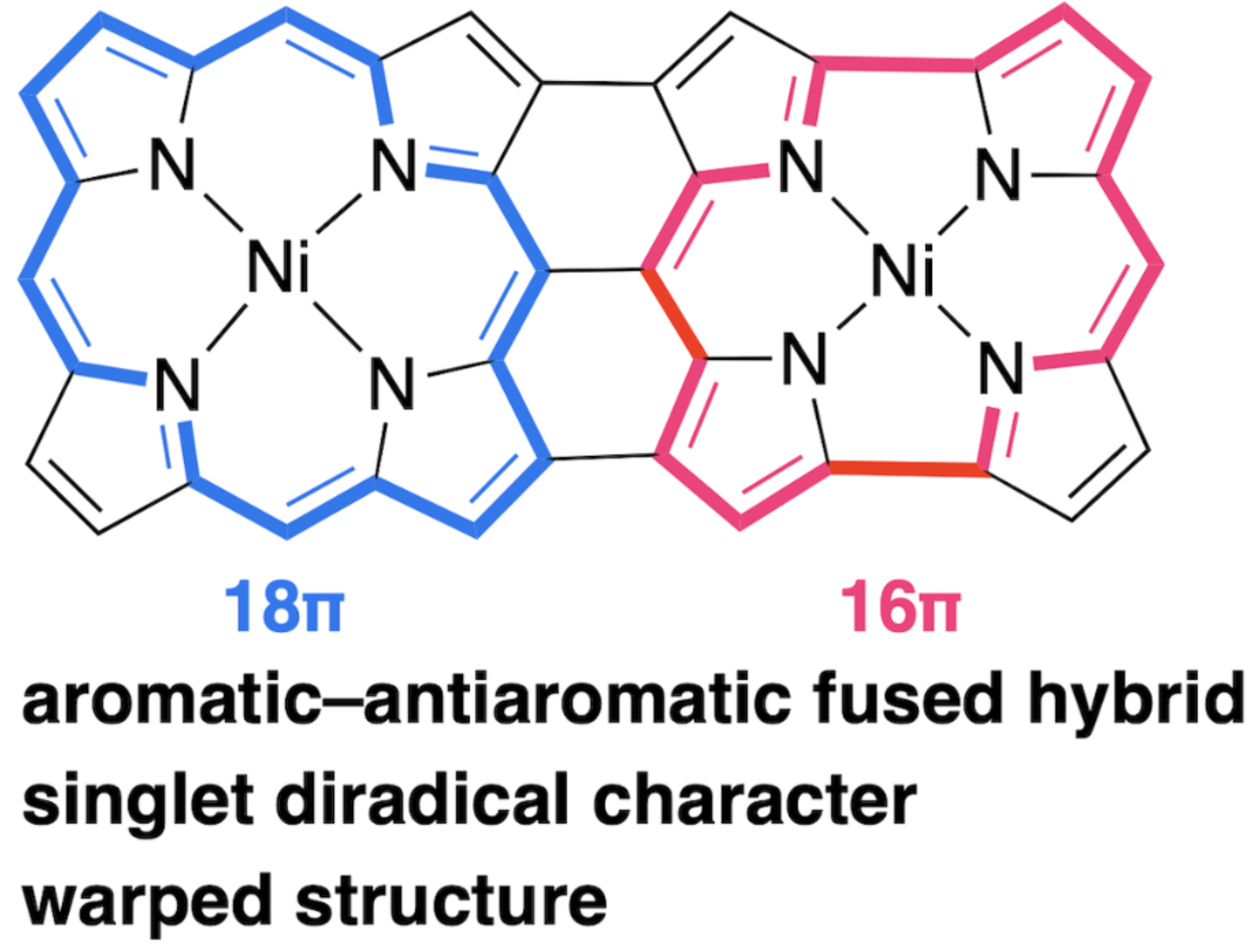
47. K. Wang, S. Ito, S. Ren, D. Shimizu, N. Fukui, R. Kishi, Q. Liu, A. Osuka, J. Song, H. Shinokubo
“A Triply-Linked Porphyrin−Norcorrole Hybrid with Singlet Diradical Character”
Angew. Chem. Int. Ed. 2024, 63, e202401233. [DOI: 10.1002/anie.202401233]
-
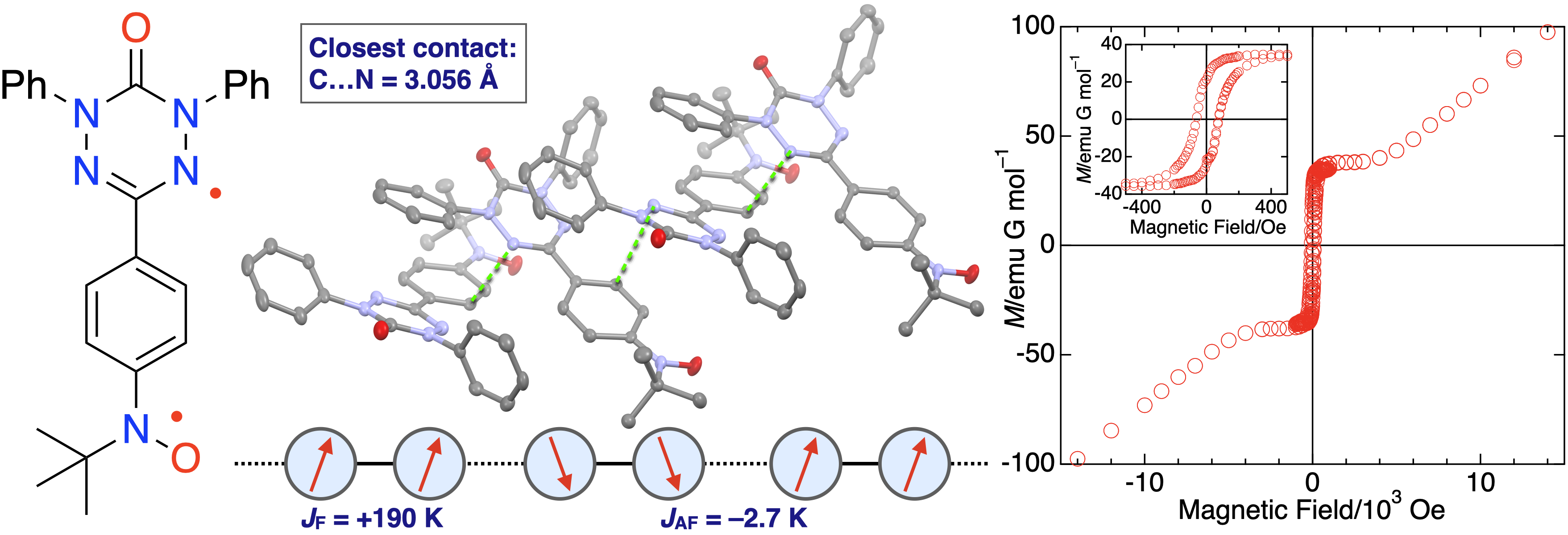
46. H. Hamamoto, D. Shimizu, K. Matsuda
“Verdazyl–Nitroxide Diradical with S = 1 Ground State: Observation of Long-Range Ordering and Haldane Gap in a Highly Isotropic S = 1 Antiferromagnetic Heisenberg Chain”
J. Phys. Chem. C 2023, 127, 21822-21828. [DOI: 10.1021/acs.jpcc.3c05584]
-
45. Y. Takeo, J. Hirano, D. Shimizu, N. Fukui, H. Shinokubo
“Effect of internal oxygen substituents on the properties of bowl-shaped aromatic hydrocarbons”
Org. Chem. Front. 2023, 10, 5895-5901. [DOI: 10.1039/D3QO01661G]

-
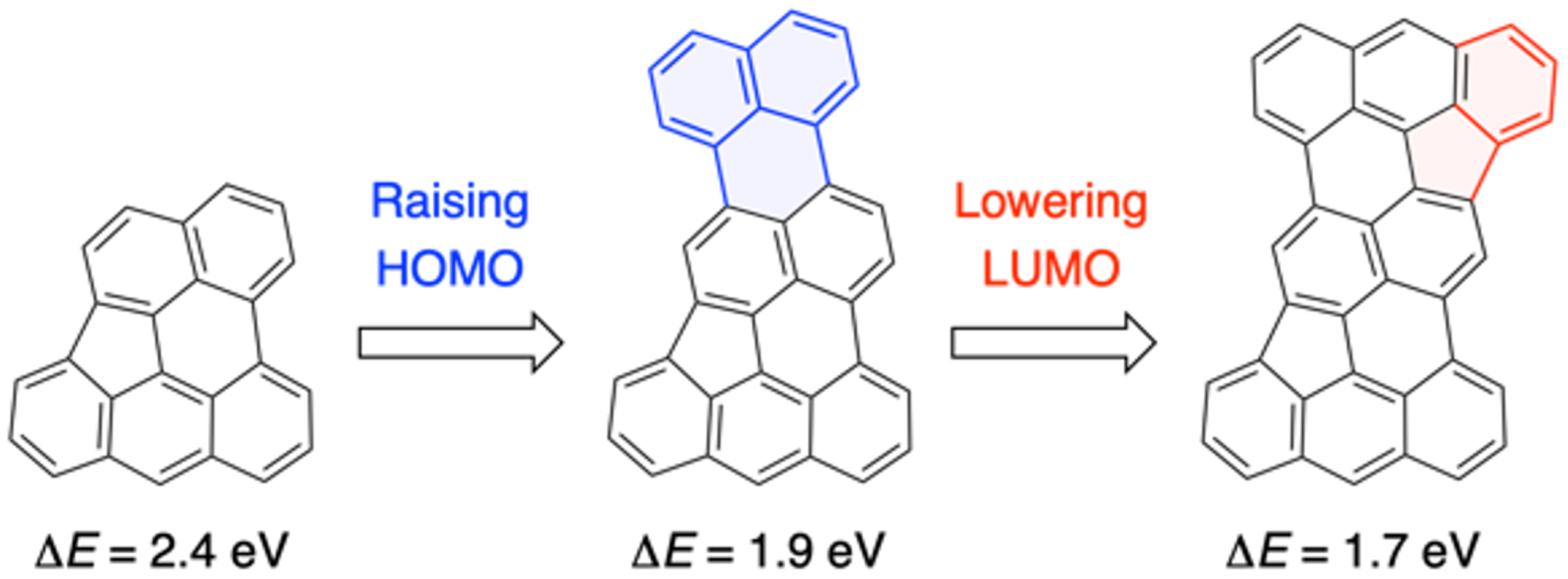
44. M. Kato, J. Kim, J. Oh, D. Shimizu, N. Fukui, H. Shinokubo
“Near-Infrared-Responsive Hydrocarbons Designed by π-Extension of Indeno[1,2,3,4-pgra]perylene at the 1,2,12-Positions”
Chem. Eur. J. 2023, 29, e202300249. [DOI: 10.1002/chem.202300249]
-
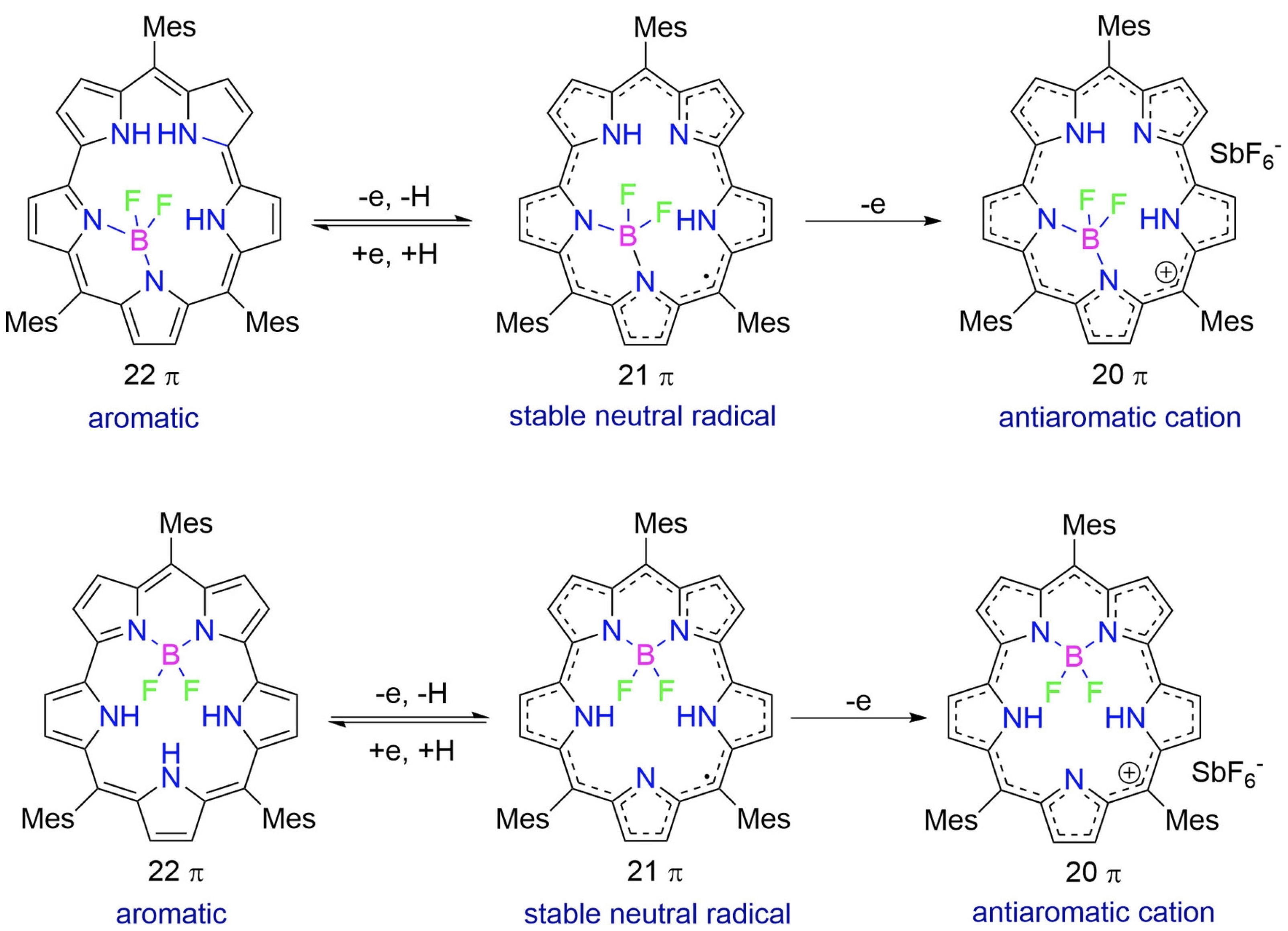
43. W. Deng, Y. Liu, D. Shimizu, T. Tanaka, A. Nakai, Y. Rao, L. Xu, M. Zhou, A. Osuka, J. Song
“Facile Formation of Stable Neutral Radicals and Cations from [22]Smaragdyrin BF2 Complexes”
Chem. Eur. J. 2023, 29, e202203484. [DOI: 10.1002/chem.202203484]
-
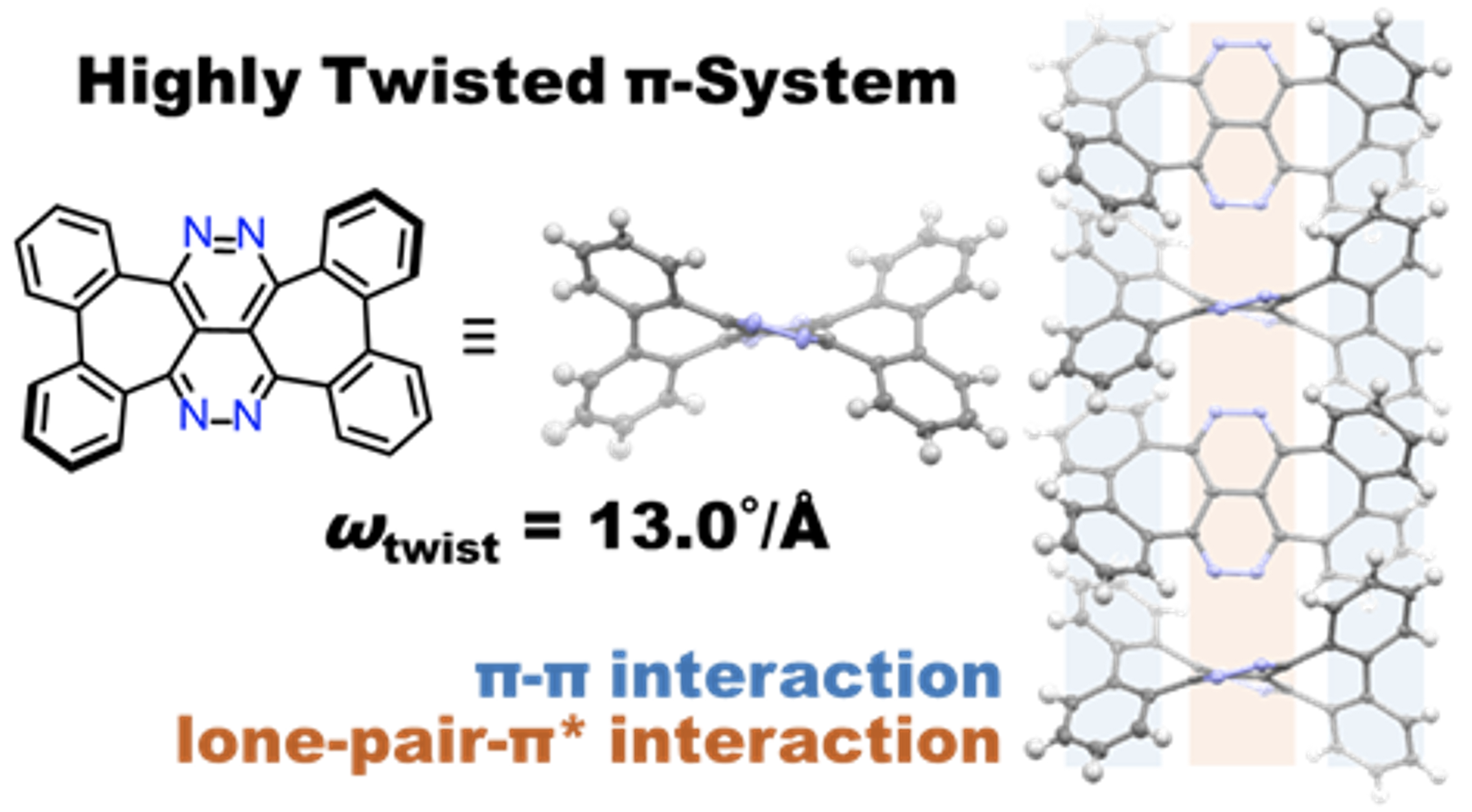
42. M. Hisada, D. Shimizu, K. Matsuda
“π-Expansion of 2,3,6,7-Tetraazanaphthalene with Two Embedded Heptagons: Highly Twisted Structure and Lone-Pair/π* Interaction in the Crystal”
Org. Lett. 2022, 24, 3707-3711. [DOI: 10.1021/acs.orglett.2c01345]
-
41. M. Hisada, D. Shimizu, K. Matsuda
“Heptagon-Embedded π-Expanded Thieno- and N-Methylpyrrolo-Pyridazines with Substantial Out-of-Plane Dipole Moment”
J. Org. Chem. 2022, 87, 9034-9043. [DOI: 10.1021/acs.joc.2c00709]
(Preprint: ChemRxiv, 10.26434/chemrxiv-2022-cnpr2).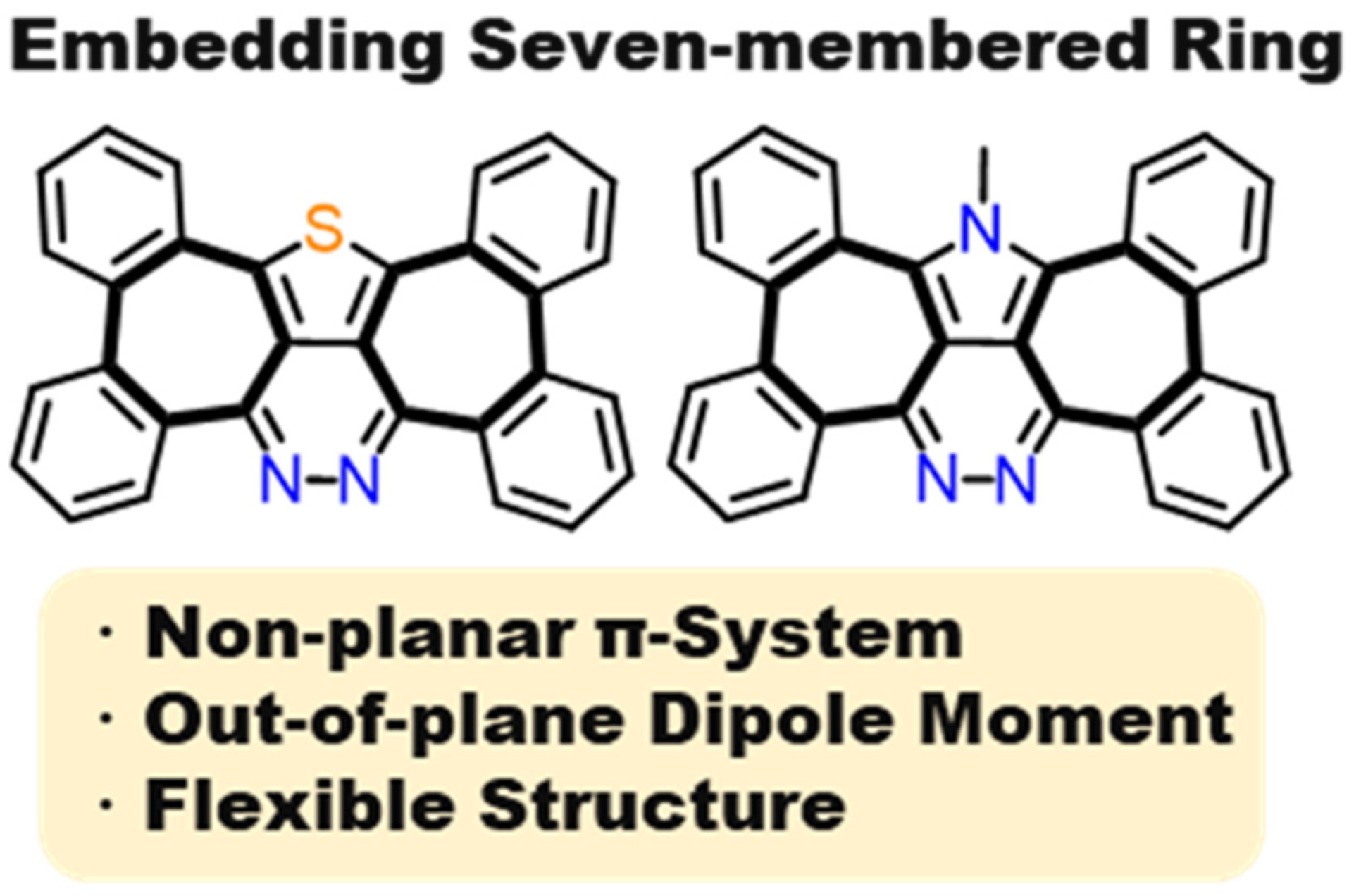
-
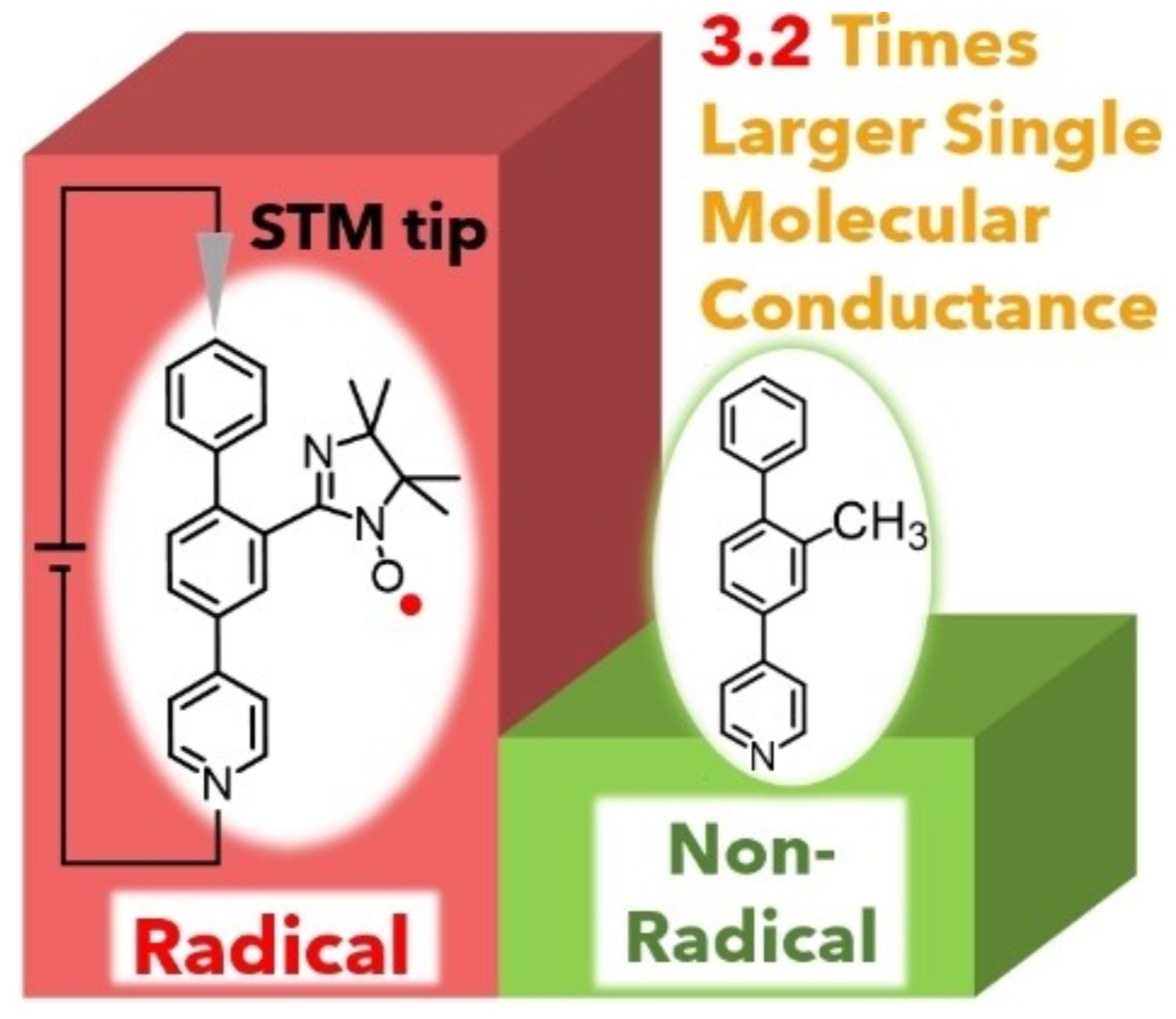
40. R. Yasui, D. Shimizu, K. Matsuda
“Large Enhancement of Single Molecular Conductance of Molecular Wire through a Radical Substituent”
Chem. Eur. J. 2022, 28, e202104242. [DOI: 10.1002/chem.202104242]

-
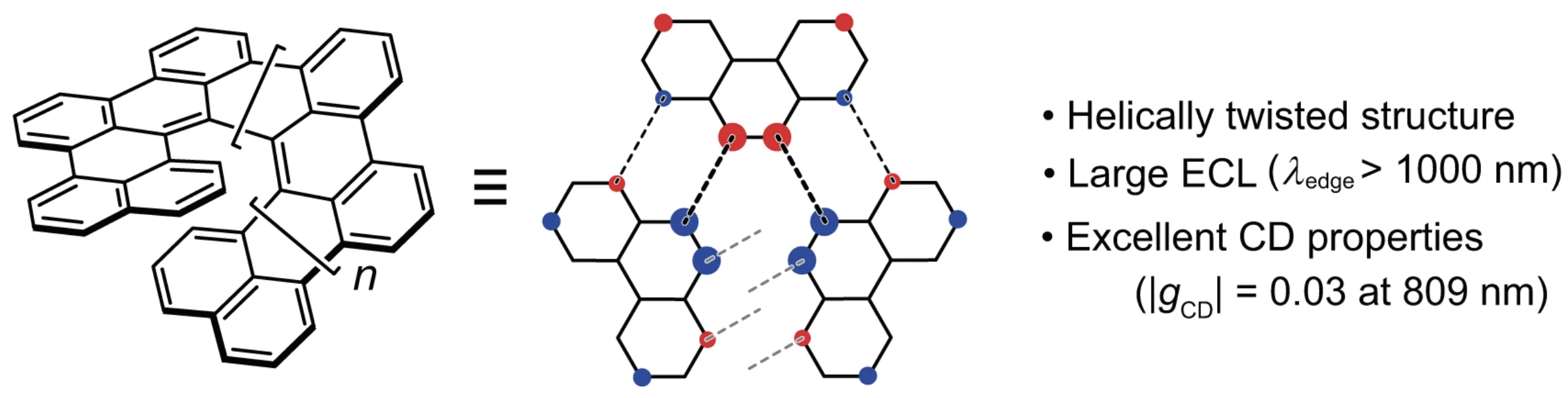
39. Y. Nakakuki, T. Hirose, H. Sotome, M. Gao, D. Shimizu, R. Li, J.-y. Hasegawa, H. Miyasaka, K. Matsuda
“Doubly linked chiral phenanthrene oligomers for homogeneously π-extended helicenes with large effective conjugation length”
Nat. Commun. 2022, 13, 1475. [DOI: 10.1038/s41467-022-29108-8] (Open access)
-
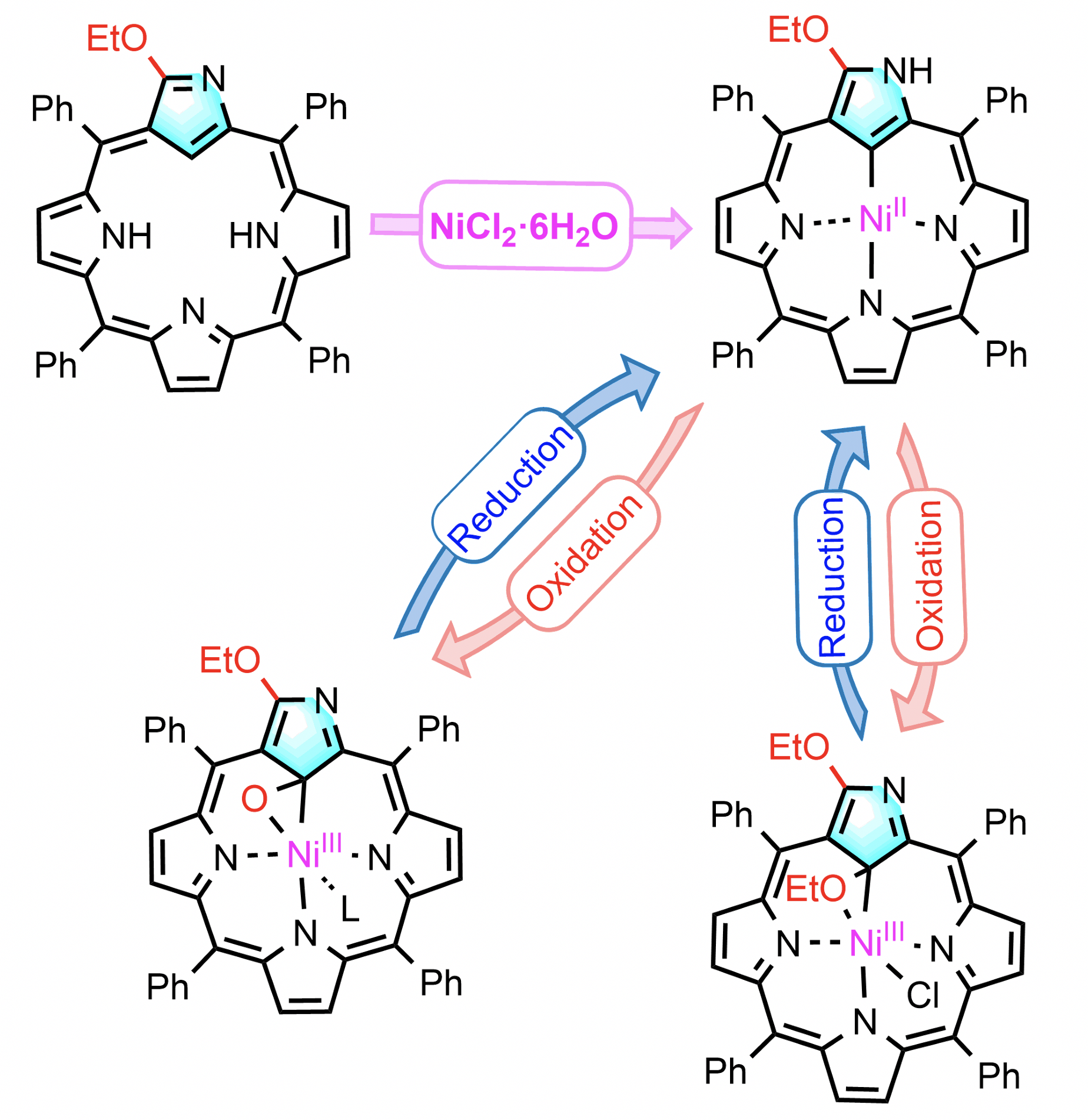
38. H. He, Z. Ye, D. Shimizu, I. Sumra, Y. Zhang, Z. Liang, Y. Zeng, L. Xu, A. Osuka, Z. Ke, H.-W. Jiang
“Formation of Stable Ni(III) N-Confused Porphyrins Aided by 3-Ethoxy Group”
Chem. Eur. J. 2022, 28, e202103272. [DOI: 10.1002/chem.202103272]
-
37. T. Shinozuka, S. Nishizawa, D. Shimizu, K. Matsuda
“Evaluation of electron transport capability of armchair graphene nanoribbons (AGNRs) by calculating exchange interaction between terminally attached radicals”
Chem. Phys. Lett. 2021, 780, 138923. [DOI: 10.1016/j.cplett.2021.138923]
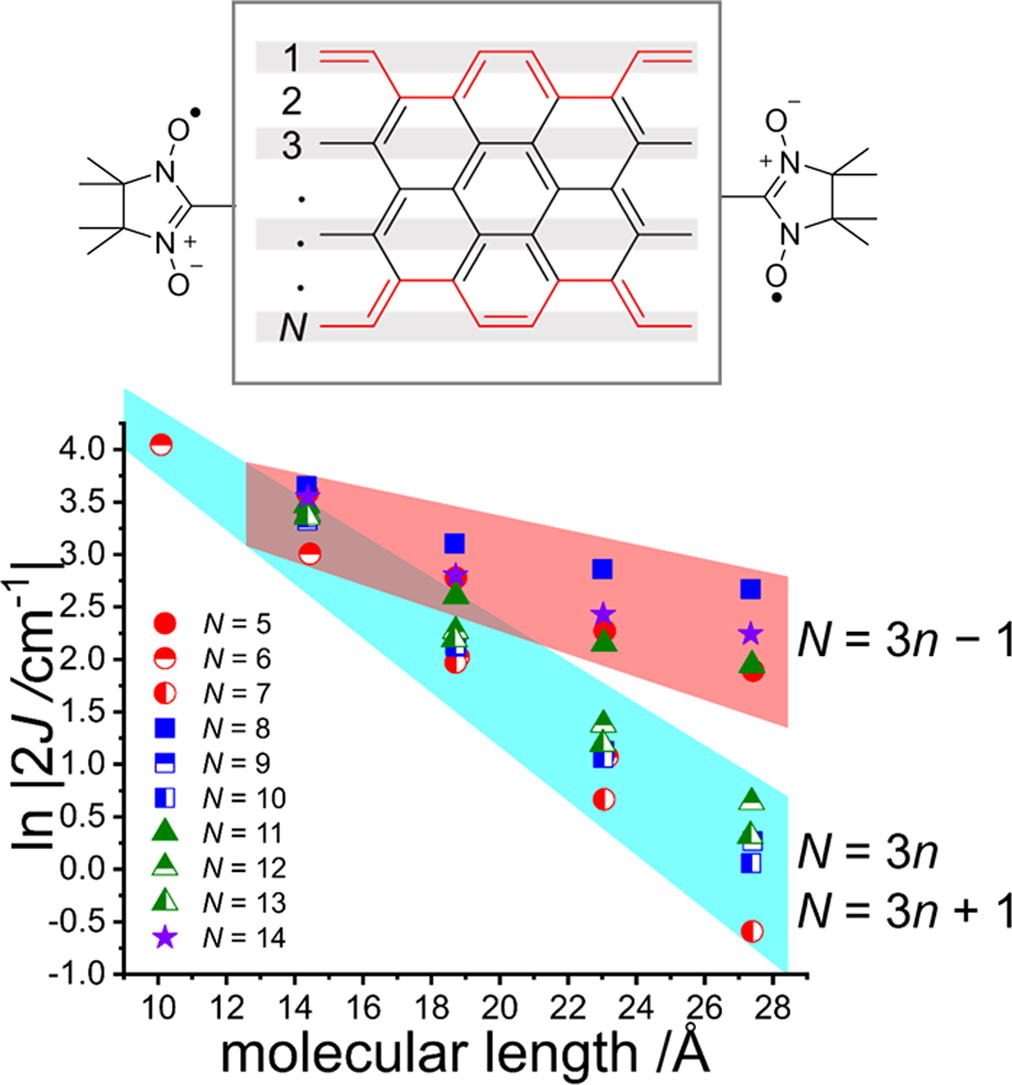
-
36. H. Kubo, T. Hirose, D. Shimizu, K. Matsuda
“Donor-Acceptor Type [5]Helicene Derivative with Strong Circularly Polarized Luminescence”
Chem. Lett. 2021, 50, 804-807. [DOI: 10.1246/cl.200913]
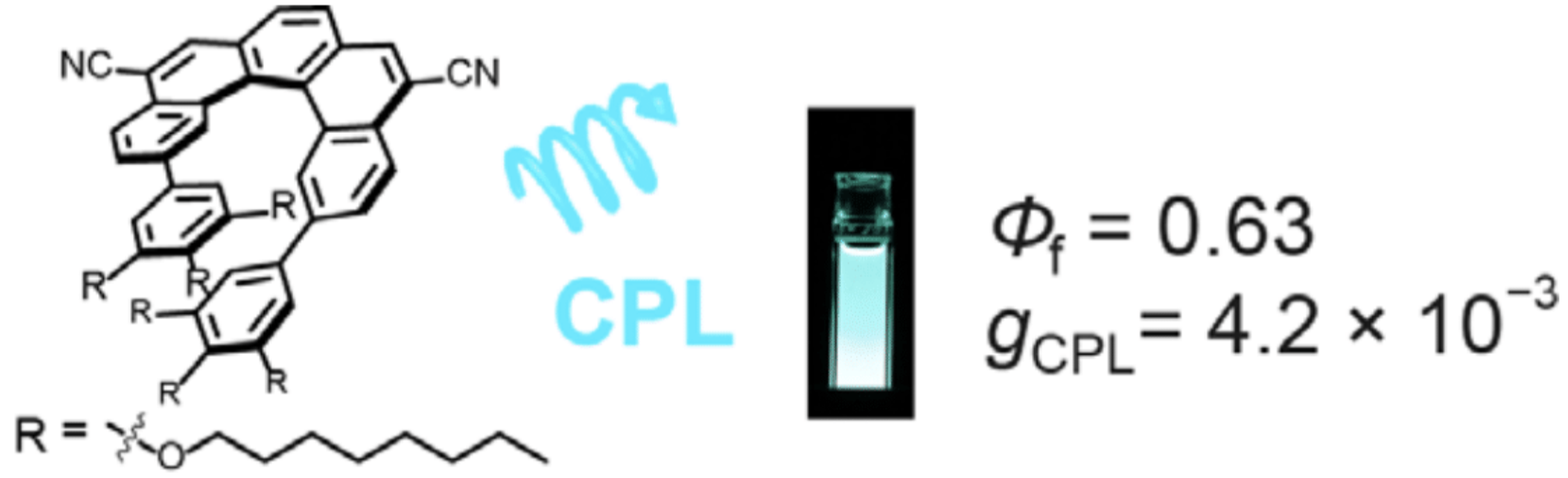
-
35. H. Kubo, D. Shimizu, T. Hirose, K. Matsuda
“Circularly Polarized Luminescence Designed from Molecular Orbit-als: A Figure-Eight-Shaped [5]Helicene Dimer with D2 Symmetry”
Org. Lett. 2020, 22, 9276-9281. [DOI: 10.1021/acs.orglett.0c03506]
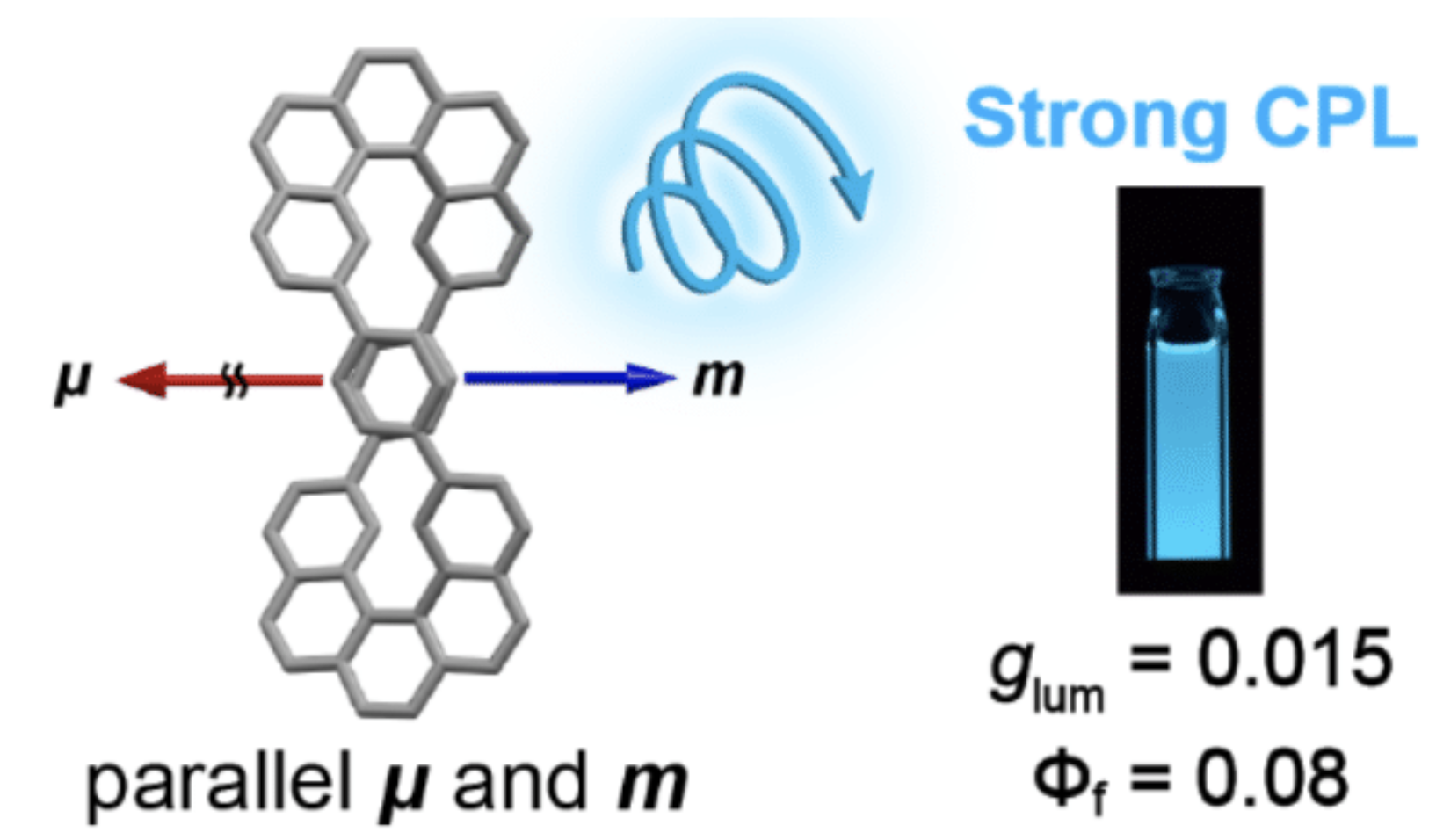
-
34.Y. J. Bae, D. Shimizu, J. D. Schultz, G. Kang, J. Zhou, G. C. Schatz, A. Osuka, M. R. Wasielewski
“Balancing Charge Transfer and Frenkel Exciton Coupling Leads to Excimer Formation in Molecular Dimers: Implications for Singlet Fission”
J. Phys. Chem. A 2020, 124, 8478-8487. [DOI: 10.1021/acs.jpca.0c07646]
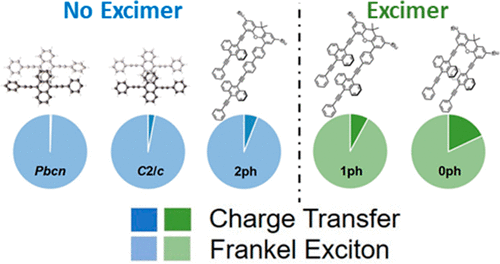
-
33. T. Iizuka, D. Shimizu, K. Matsuda
“STM apparent height measurements of molecular wires with different physical length attached on 2-D phase separated templates for evaluation of single molecular conductance”
RSC Adv. 2020, 10, 22054-22057. [DOI: 10.1039/D0RA04484A]
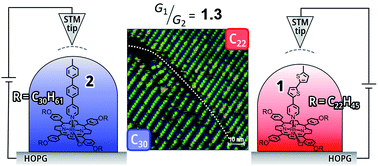
-
32. S. Ooi, B. Adinarayana, D. Shimizu, T. Tanaka, and A. Osuka
“Stable meso-meso Linked 2NH-Corrole Radical Dimers as a Key Intermediate to Corrole Tape”
Angew. Chem. Int. Ed. 2020, 59, 9423-9427. [DOI: 10.1002/anie.202002976]
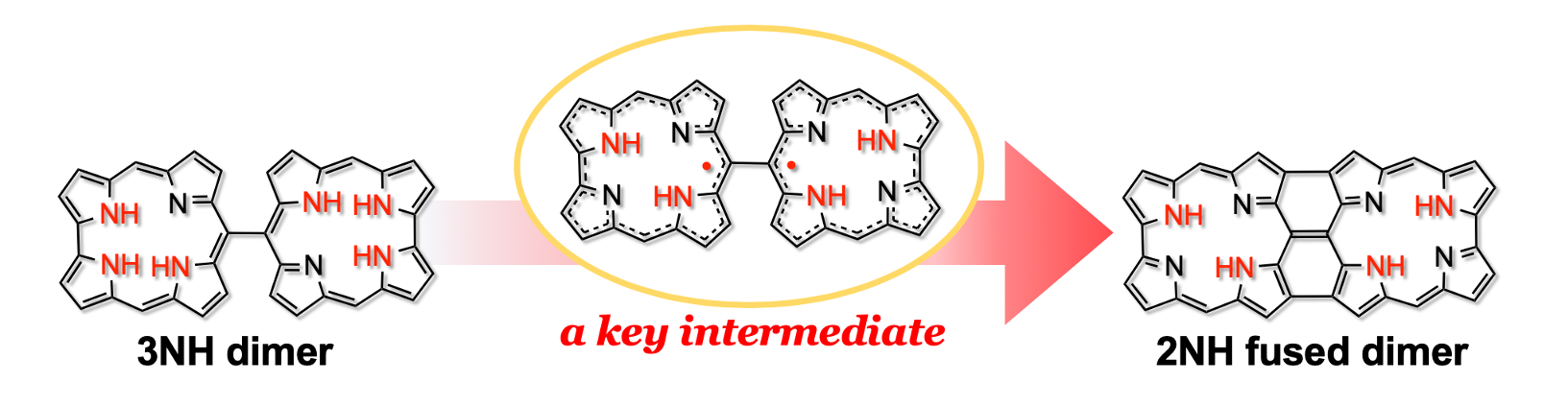
-
31. M. Izawa, T. Suito, S. Ishida, D. Shimizu, T. Tanaka, T. Mori, A. Osuka
“Figure-eight Octaphyrin Bis-Ge(IV) Complexes: Synthesis, Structures, Aromaticity, and Chiroptical Properties”
Chem. Asian J. 2020, 15, 1440-1448. [DOI: 10.1002/asia.202000159]
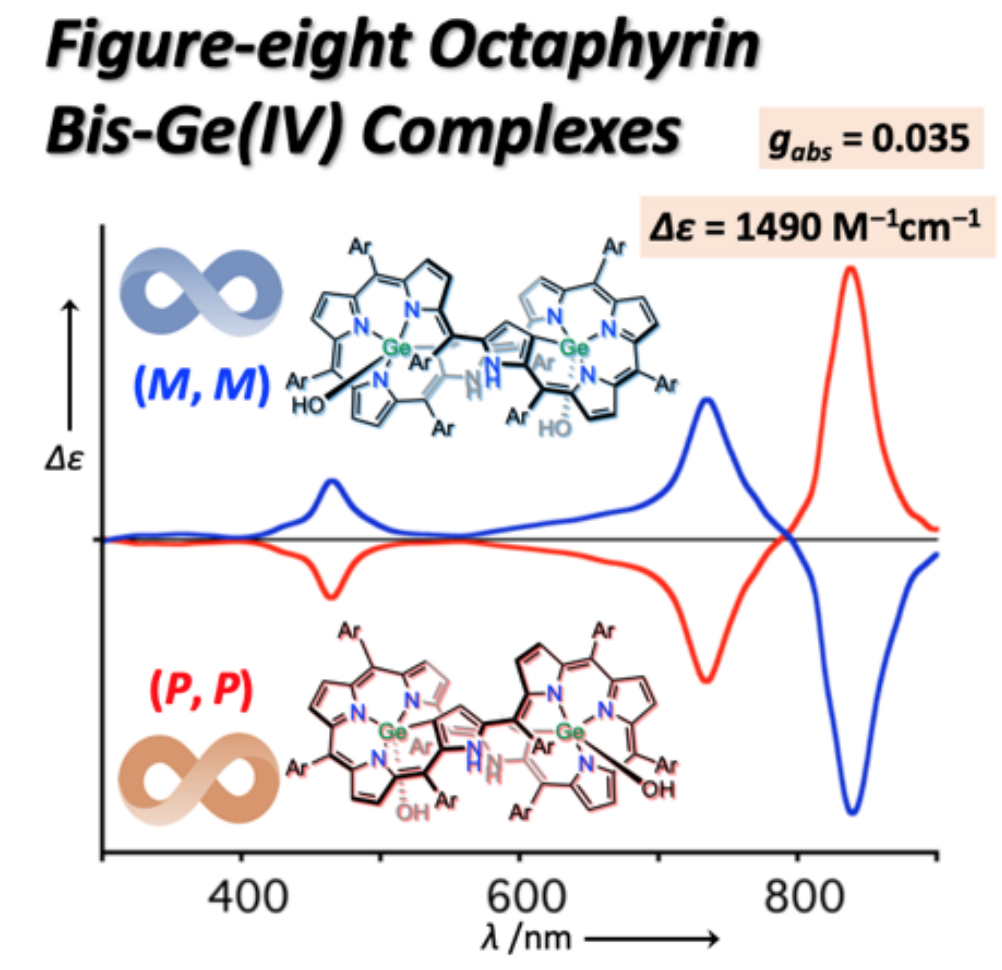
-
30. G. Lavarda, D. Shimizu, T. Torres, A. Osuka
“meso-(2-Pyridyl)-boron(III)-subporphyrin: Perimeter Iridium(III) Coordination”
Angew. Chem. Int. Ed. 2020, 59, 3127-3130. [DOI: 10.1002/anie.201914853]
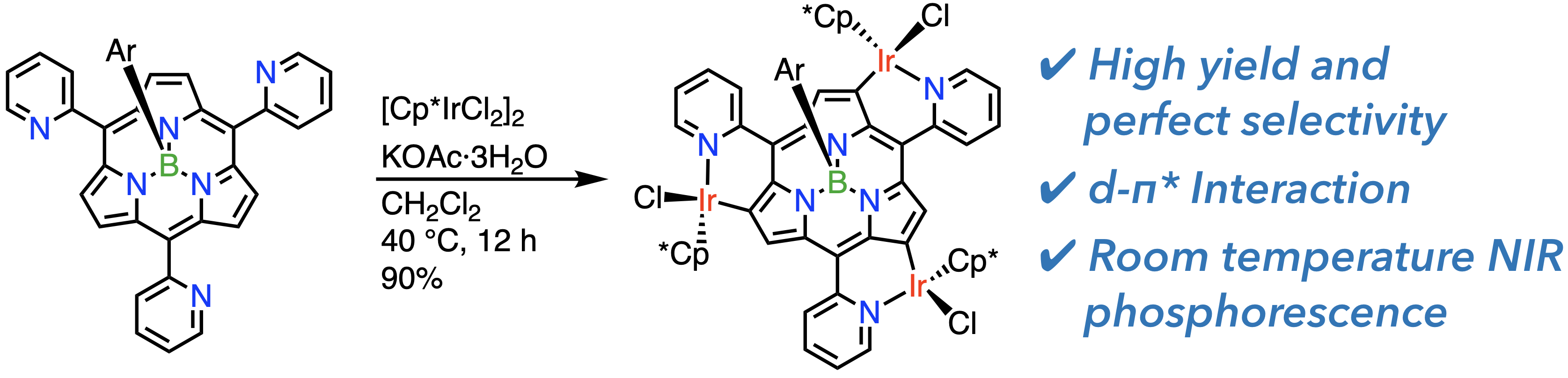
-
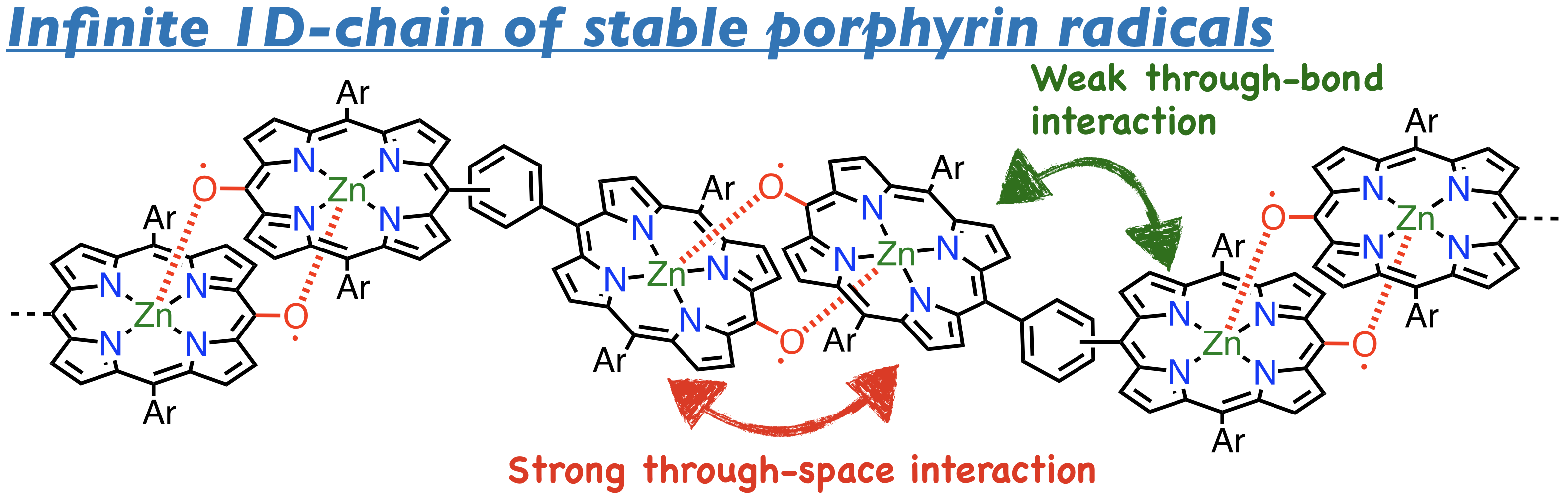
29. B. Adinarayana, K. Kato, D. Shimizu, K. Furukawa, T. Tanaka, A. Osuka
“Cyclophane-type Chlorin Dimers from Dynamic Covalent Chemistry of 2,18-Porphyrinyl Dicyanomethyl Diradicals”
Angew. Chem. Int. Ed. 2020, 4320-4323. [DOI: 10.1002/anie.201914480]
-
28. T. Yamamoto, K. Kato, D. Shimizu, T. Tanaka, A. Osuka
“Phenylene-bridged Porphyrin meso-Oxy Radical Dimers”
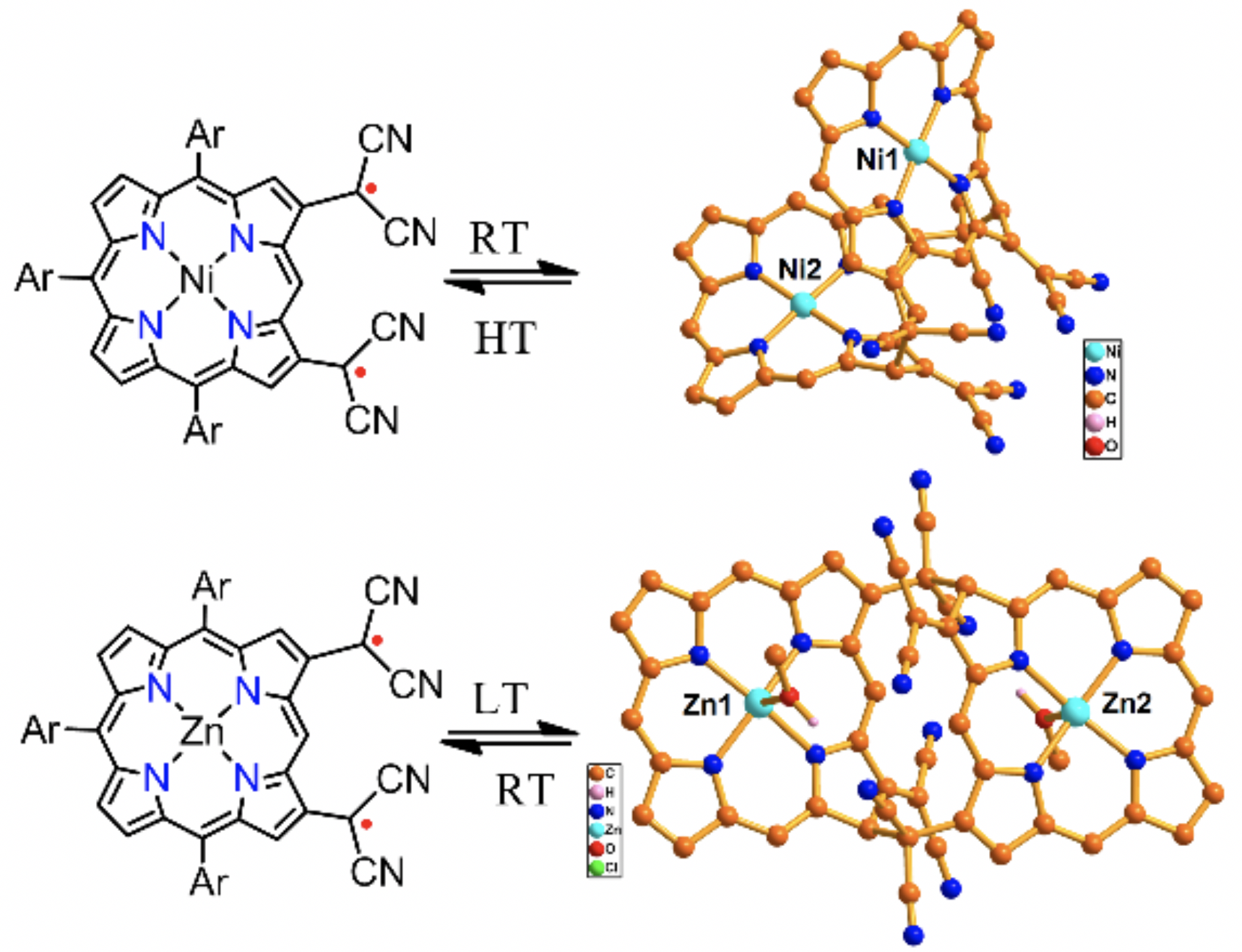
Chem. Asian J. 2019, 14, 4031-4034. [DOI: 10.1002/asia.201901033]
-
27. C. Schierl, W. Alex, L. M. Mateo, B. Ballesteros, D. Shimizu, A. Osuka, T. Torres, D. M. Guldi, G. Bottari
“Quadrupolar Cyclopenta[hi]aceanthrylene‐based Electron Donor‐Acceptor‐Donor Conjugates: Charge Transfer versus Charge Separation”
Angew. Chem. Int. Ed. 2019, 58, 14644-14652. [DOI: 10.1002/anie.201906206]
-
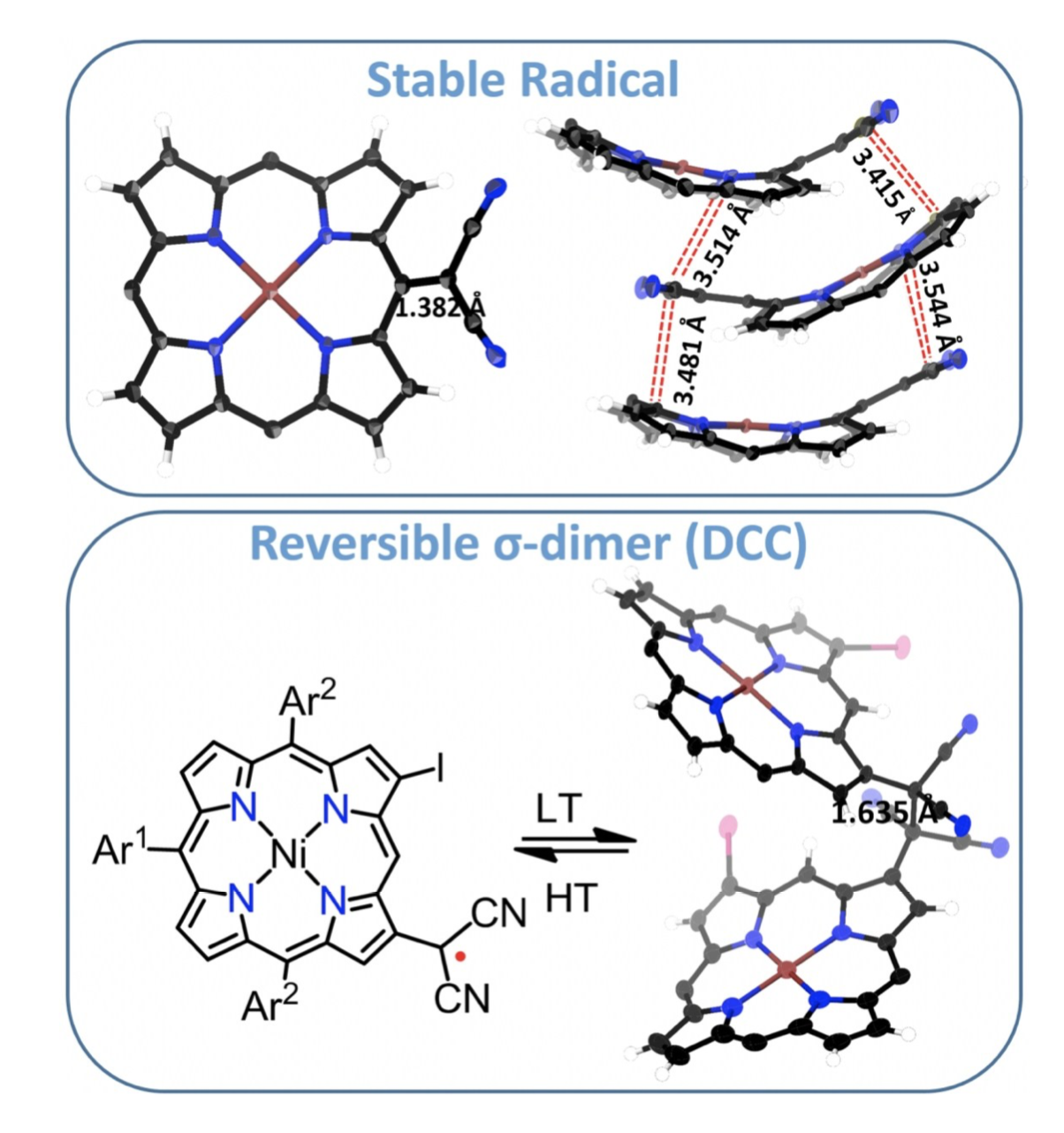
26. B. Adinarayana, D. Shimizu, K. Furukawa, A. Osuka
“Stable radical versus reversible σ-bond formation of (porphyrinyl)dicyanomethyl radicals”
Chem. Sci. 2019, 10, 6007-6012. [DOI: 10.1039/C9SC01631G] (Open access)
-
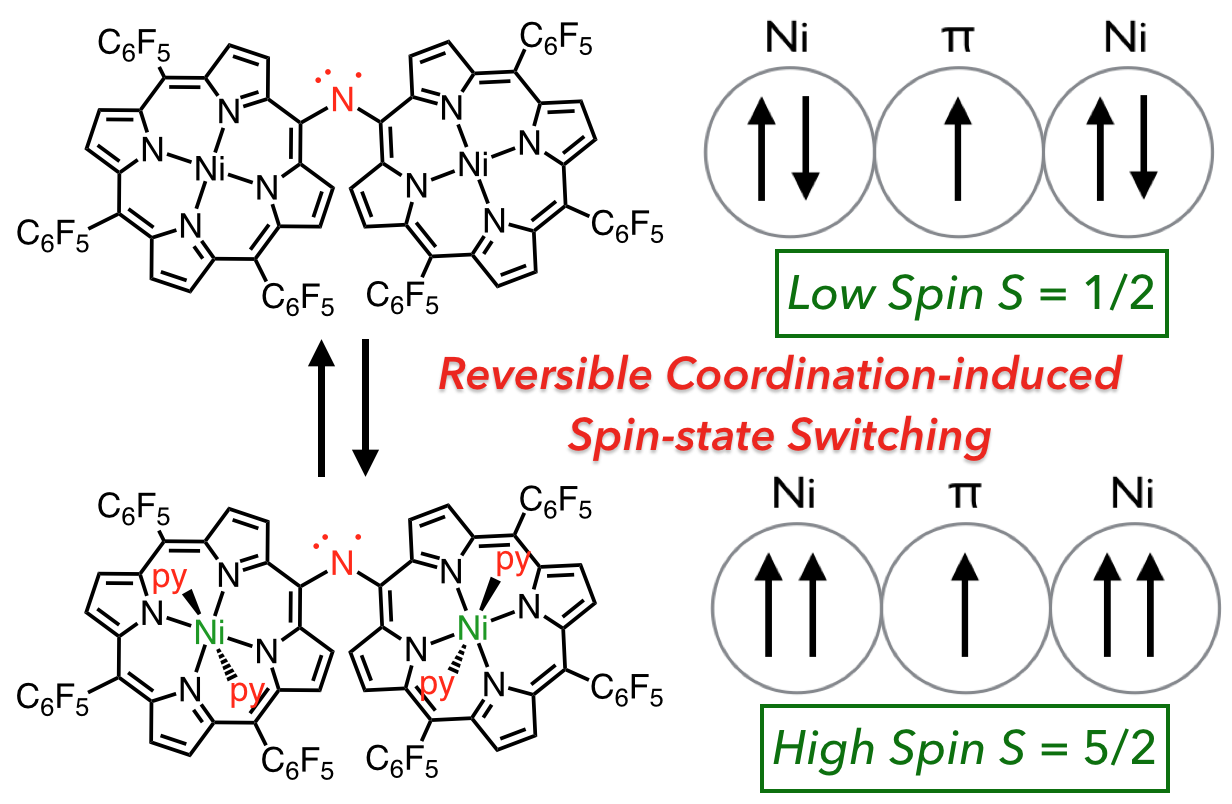
25. D. Shimizu, Y. Ide, T. Ikeue, A. Osuka
“Coordination-Induced Spin-State Switching of an Aminyl-Radical-Bridged Nickel(II) Porphyrin Dimer between Doublet and Sextet States”
Angew. Chem. Int. Ed. 2019, 58, 5023-5027. [DOI: 10.1002/anie.201900792]
-
24. K. Fujimoto, D. Shimizu, T. Mori, Y. Li, M. Zhou, J. Song, A. Osuka
“Selective Formation of Helical Tetrapyrrin-fused Porphyrins by Oxidation of β-to-β Linked meso-Aminoporphyrin Dimers”
Chem. Eur. J. 2019, 25, 1711-1715. [DOI: 10.1002/chem.201805659]
-
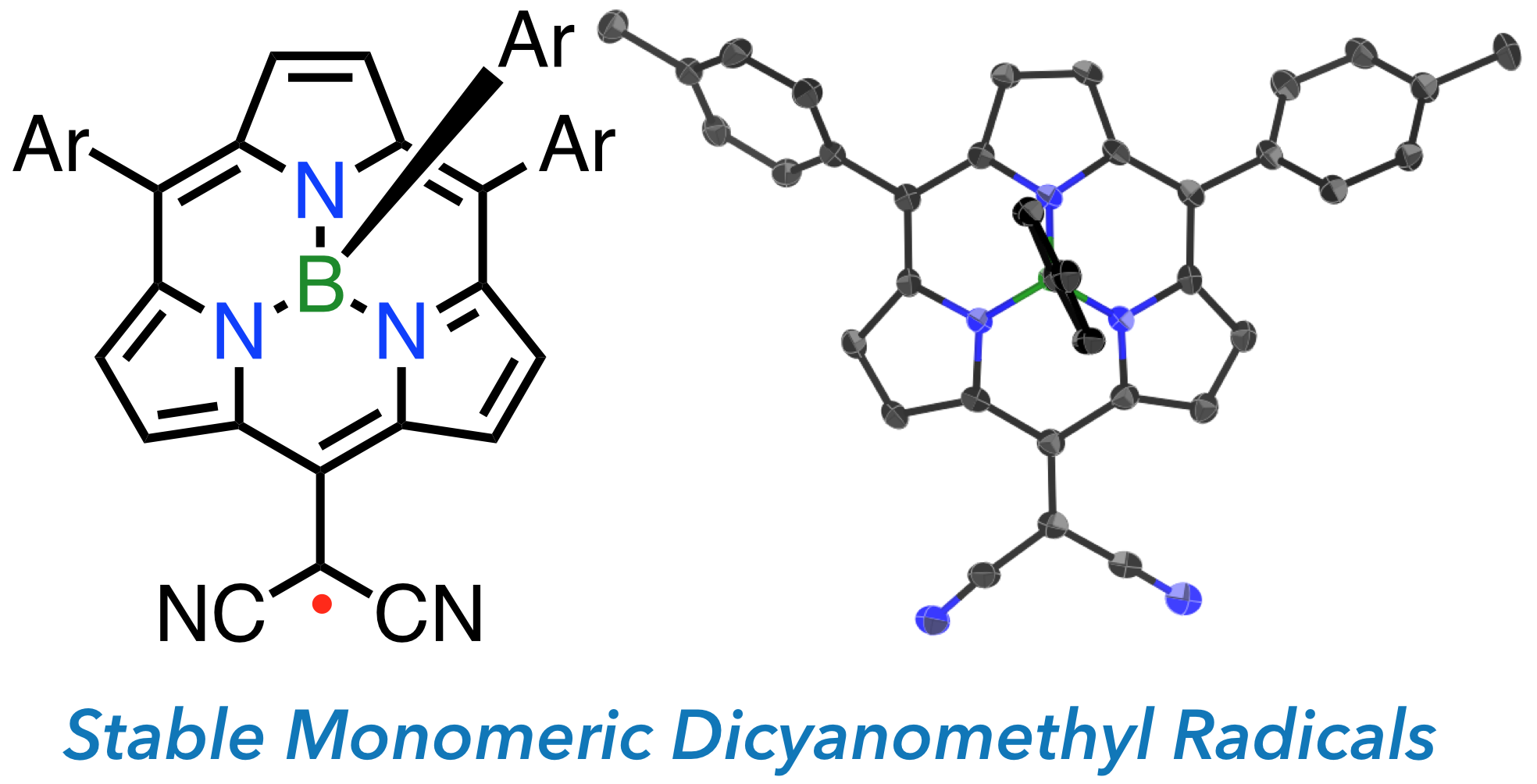
23. B. Adinarayana, D. Shimizu, A. Osuka
“Stable (BIII-Subporphyrin-5-yl)dicyanomethyl Radicals”
Chem. Eur. J. 2019, 25, 1706-1710. [DOI: 10.1002/chem.201805601]
-
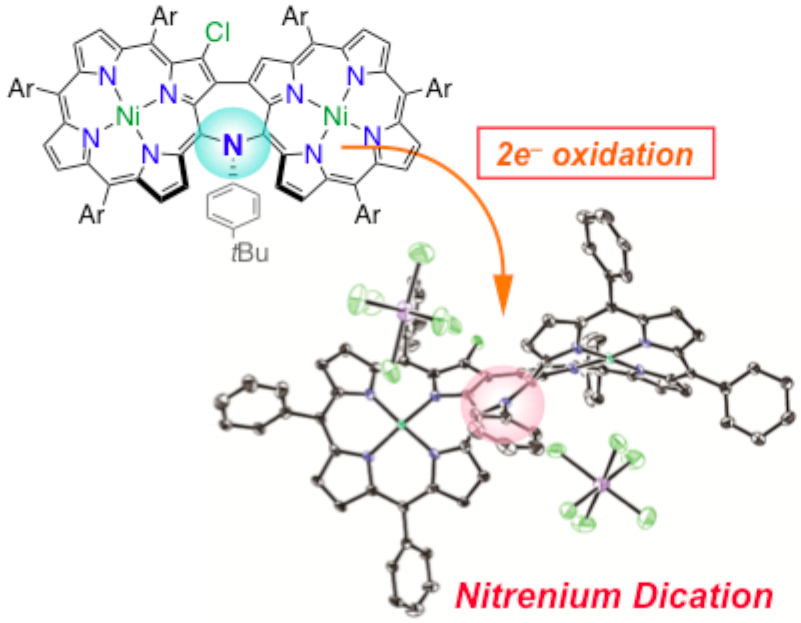
22. K. Fujimoto, D. Shimizu, A. Osuka
“Porphyrin-Stabilized Nitrenium Dication”
Chem. Eur. J. 2019, 25, 521-525. [DOI: 10.1002/chem.201805491]
-
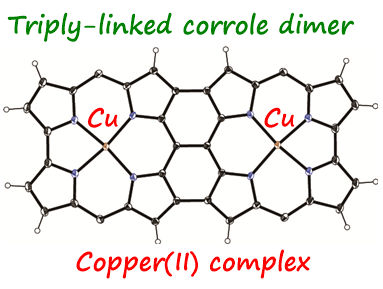
21. S. Ooi, T. Tanaka, T. Ikeue, K. Yamasumi, K Ueta, D Shimizu, M. Ishida, H. Furuta, A. Osuka
“Bis-copper(II) Complex of Triply-linked Corrole Dimer and Its Dication”
Chem. Asian J. 2019, 14, 1771-1776. [DOI: 10.1002/asia.201801467]
-
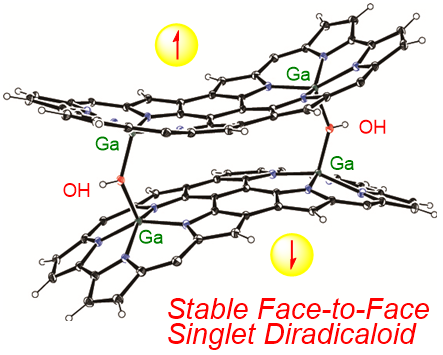
20. S. Ooi, D. Shimizu, K. Furukawa, T. Tanaka, A. Osuka
“Stable Face‐to‐Face Singlet Diradicaloids: Triply Linked Corrole Dimer Gallium(III) Complexes with Two μ‐Hydroxo‐Bridges”
Angew. Chem. Int. Ed. 2018, 57, 14916-14920. [DOI: 10.1002/anie.201810200]
-
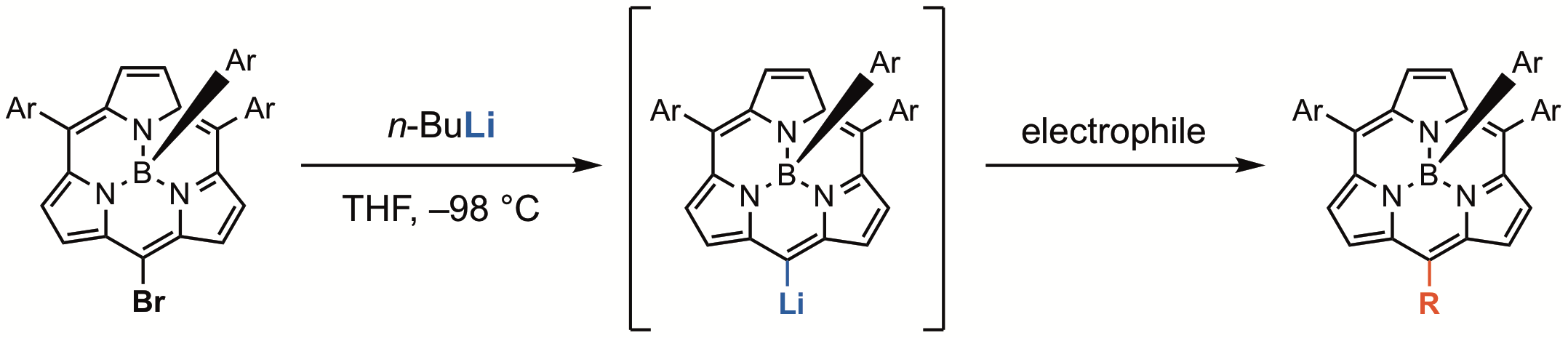
19. Y. Bekki, D. Shimizu, K. Fujimoto, A. Osuka
“meso-Functionalizations of BIII Subporphyrin with BIII meso-Lithiosubporphyrin”
Chem. Eur. J. 2018, 24, 12708-12715. [DOI: 10.1002/chem.201802339]
-
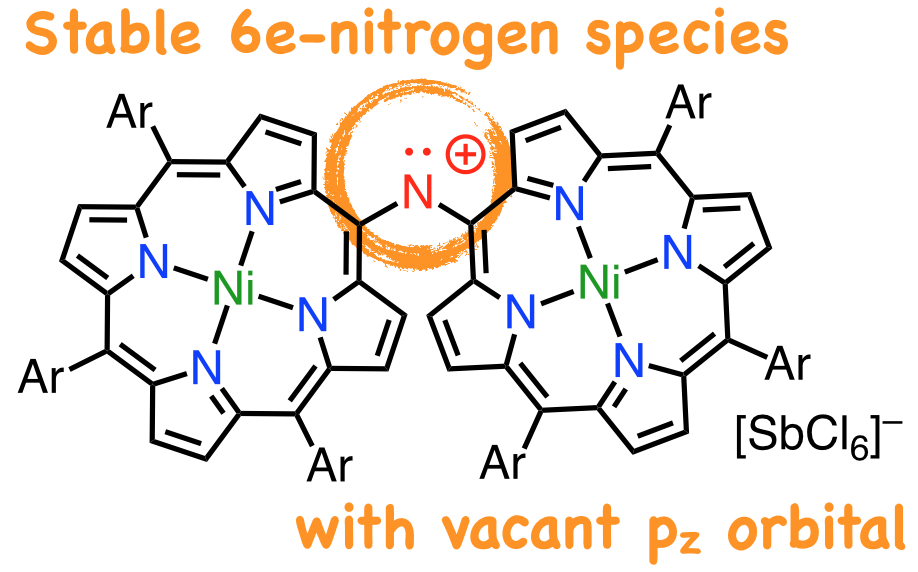
18. D. Shimizu, K. Fujimoto, A. Osuka
“Stable Diporphyrinyl-Aminyl Radical and Nitrenium Ion”
Angew. Chem. Int. Ed. 2018, 57, 12708-12715. [DOI: 10.1002/anie.201805385]
-
17. K. Kise, Y. Hong, N. Fukui, D. Shimizu, D. Kim, A. Osuka
“Diarylamine-fused Subporphyrins: Proof of Twisted Intramolecular Charge Transfer (TICT) Mechanism”
Chem. Eur. J. 2018, 24, 8306-8310. [DOI: 10.1002/chem.201801576]
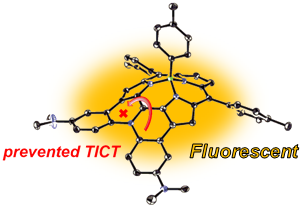
-
16. K. Kise, K. Yoshida, R. Kotani, D. Shimizu, A. Osuka
“BIII 5-Arylsubporphyrins and BIII subporphine”
Chem. Eur. J. 2018, 24, 19136-19140. [DOI: 10.1002/chem.201801491]
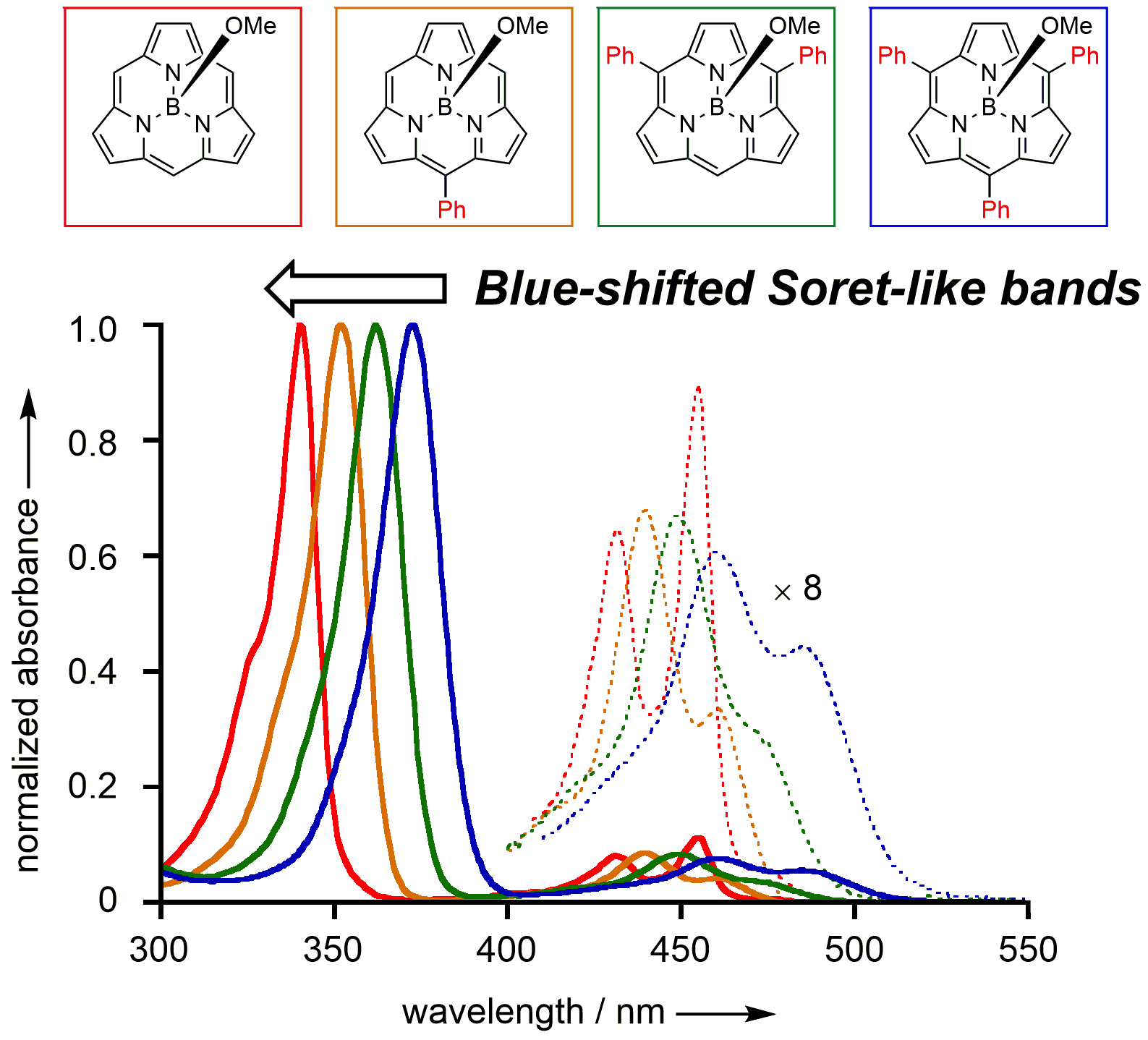
-
15. S. Ishida, J. Kim, D. Shimizu, D. Kim, A. Osuka
“Synthesis of bis-Silicon Complexes of [38]-, [37]-, and [36] Octaphyrins: Aromaticity Switch and Stable Radical Cation”
Angew. Chem. Int. Ed. 2018, 57, 5876-5880. [DOI: 10.1002/anie.201801986]
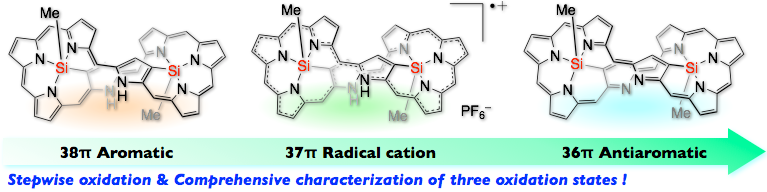
-
14. D. Shimizu, A. Osuka
“A Benzene-1,3,5-Triaminyl Radical Fused with Zn(II)-Porphyrins: Remarkable Stability and High Spin Quartet Ground State”
Angew. Chem. Int. Ed. 2018, 57, 3733-3736. [DOI: 10.1002/anie.201801080]
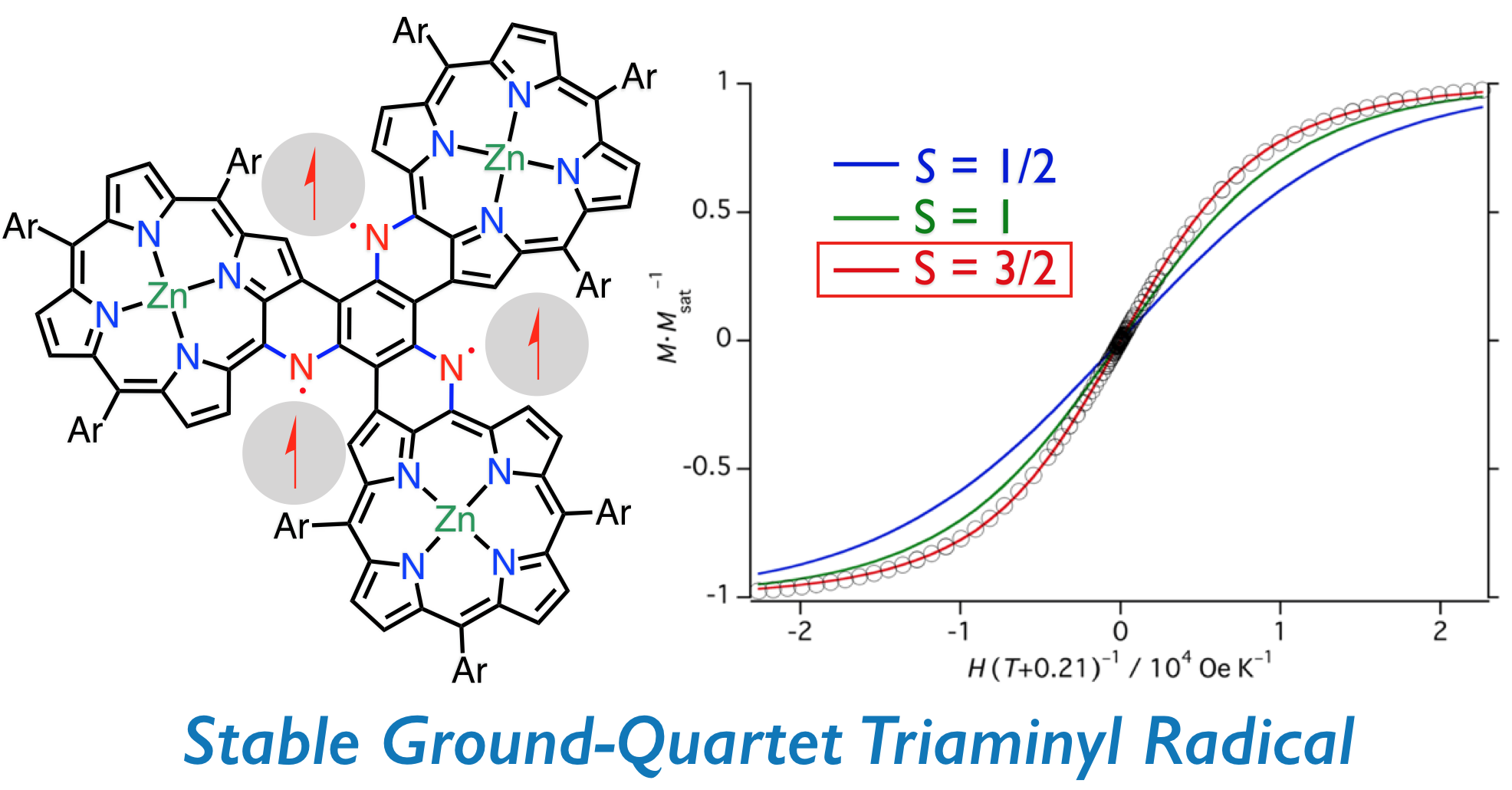
-
13. D. Shimizu, K Furukawa, A. Osuka
“Stable Subporphyrin meso-Aminyl Radicals without Resonance Stabilization by Neighboring Heteroatom”
Angew. Chem. Int. Ed. 2017, 56, 7435-7439. [DOI: 10.1002/anie.201703097]
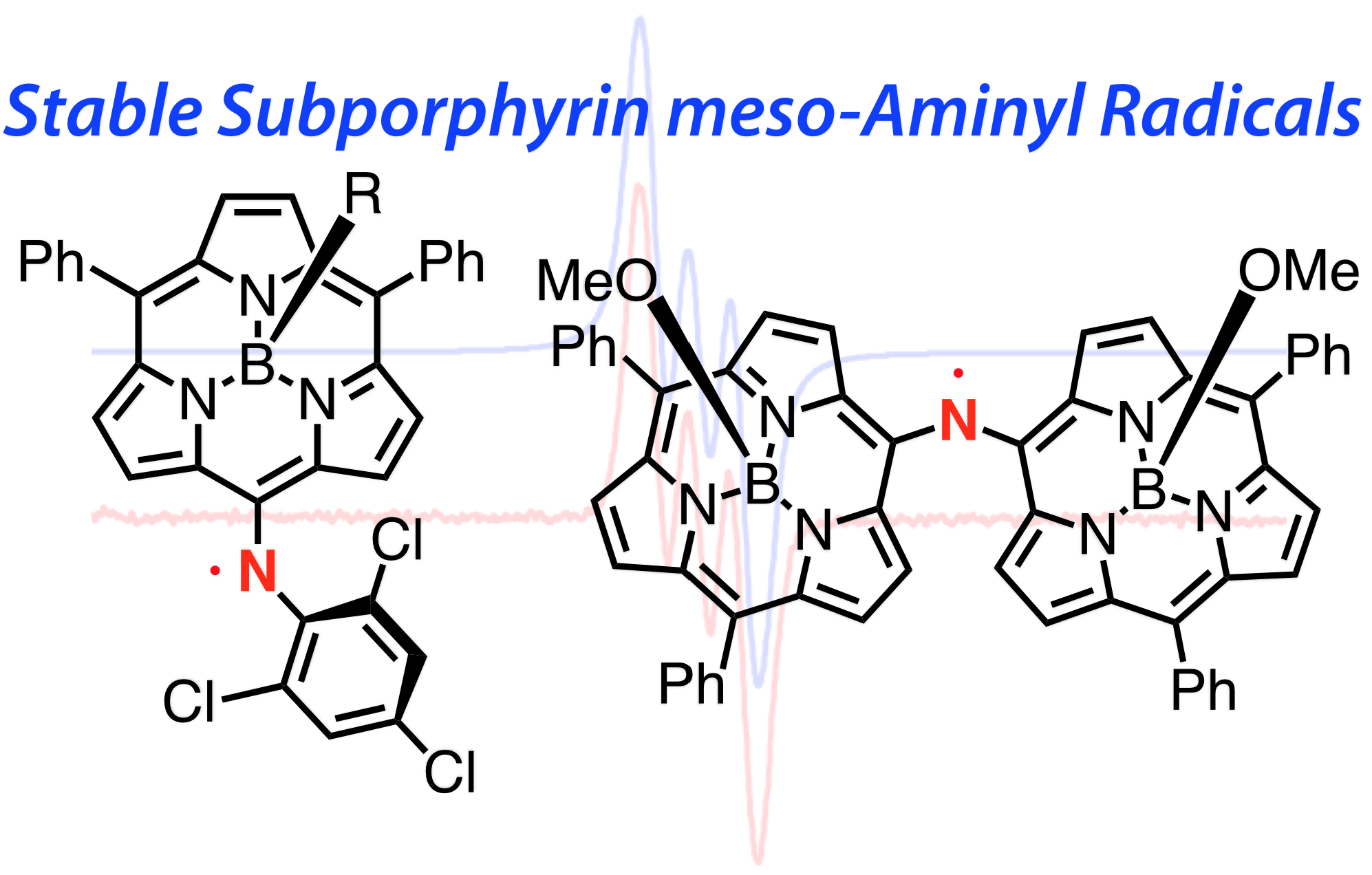
-
12. K. Naoda, D. Shimizu, J. O. Kim, K. Furukawa, D. Kim, A. Osuka
“Thienylquinonoidal Porphyrins and Hexaphyrins with Singlet Diradical Ground States”
Chem. Eur. J. 2017, 23, 8969-8879. [DOI: 10.1002/chem.201701355]
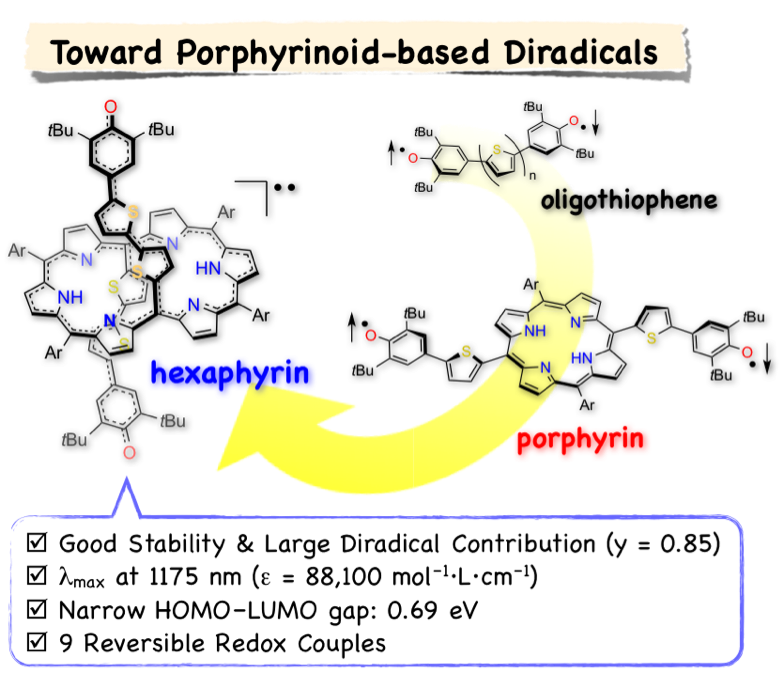
-
11. C. Stähler, D. Shimizu, K. Yoshida, K. Furukawa, R. Herges, A. Osuka
“Stable NiII Porphyrin meso-Oxy Radical with a Quartet Ground State”
Chem. Eur. J. 2017, 23, 7217-7200. [DOI: 10.1002/chem.201701354]
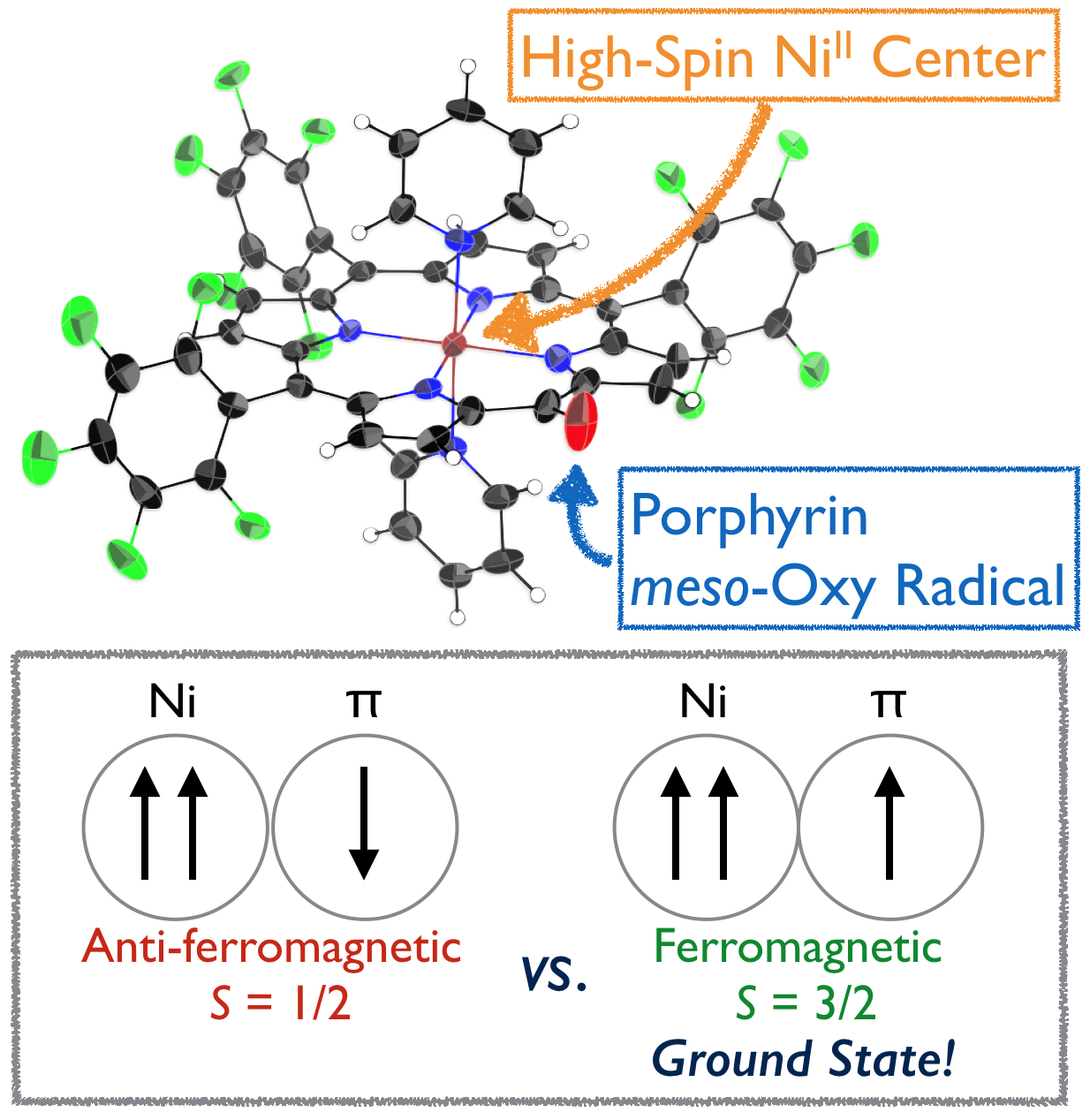
-
10. D. Shimizu, S.-K. Lee, D. Kim, A. Osuka
“meso-Nitro- and meso-Aminosubporphyrinatoboron(III)s and meso-to-meso-Azosubporphyrinatoboron(III)s”
Chem. Asian J. 2016, 11, 2946-2952. [DOI: 10.1002/asia.201601019]
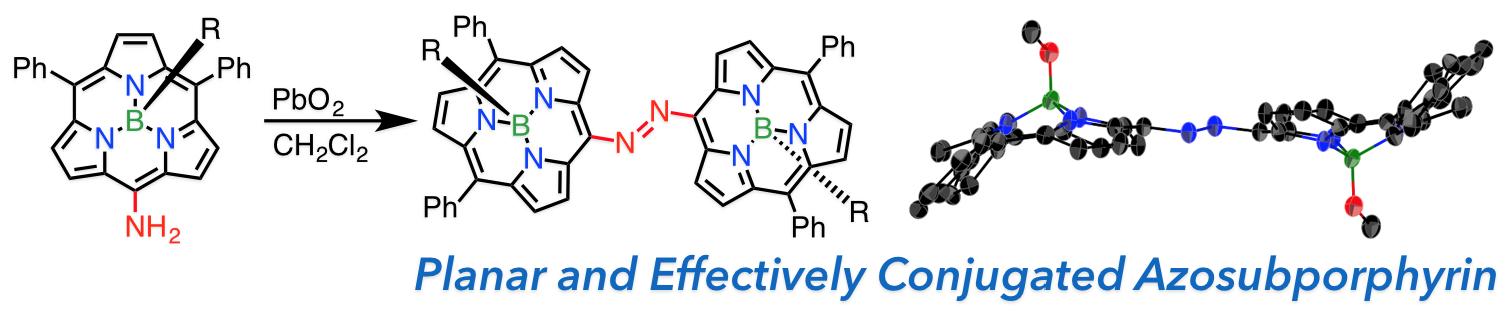
-
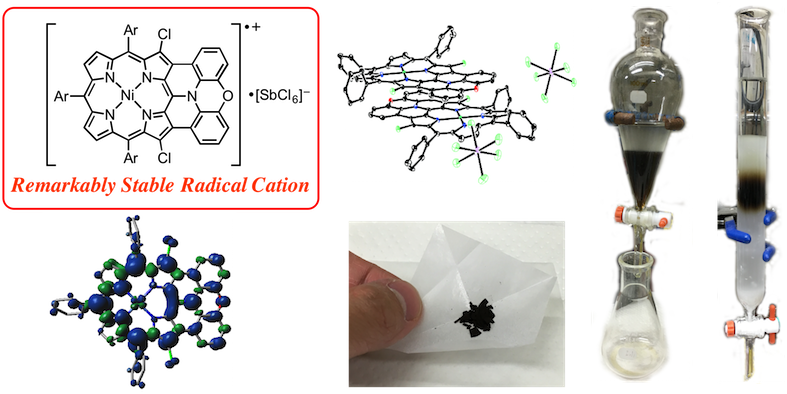
9. N. Fukui, W. Cha, D. Shimizu, J. Oh, K. Furukawa, H. Yorimitsu, D. Kim, A. Osuka
“Highly planar diarylamine-fused porphyrins and their remarkably stable radical cations”
Chem. Sci. 2017, 8, 189-199. [DOI: 10.1039/C6SC02721K] (Open access)
-
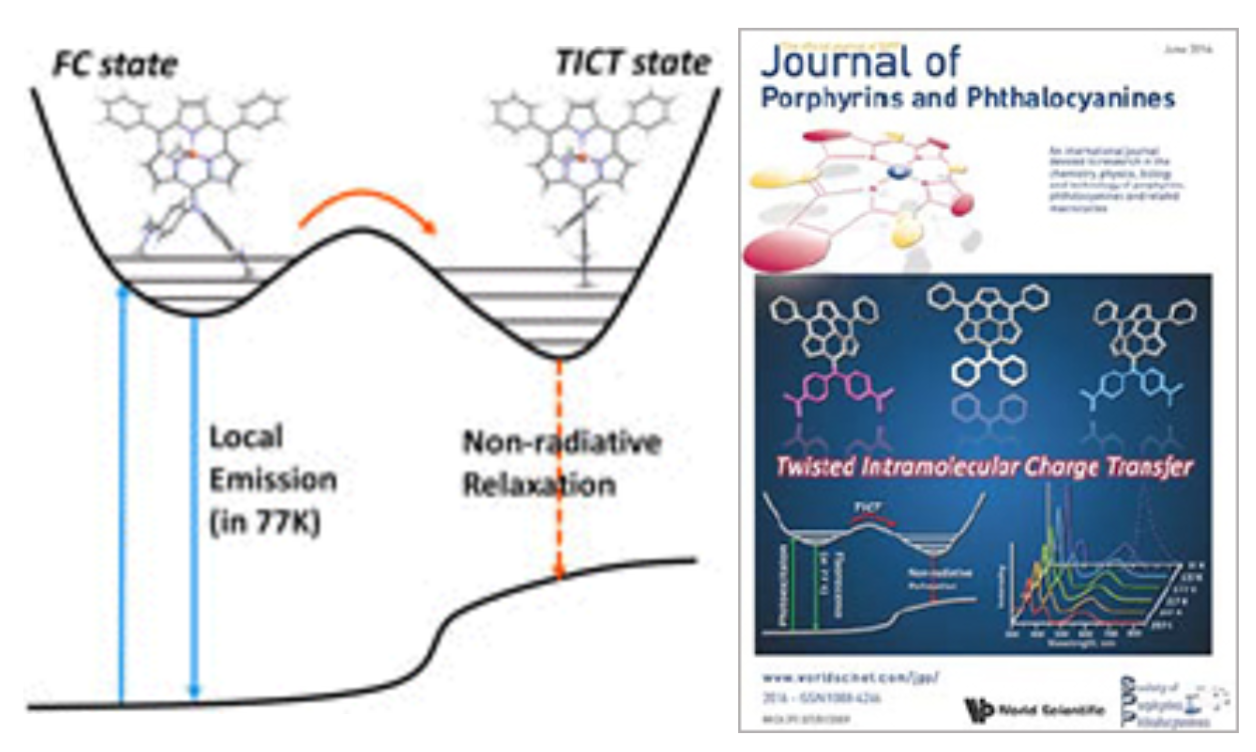
8. S.-K. Lee, J. O. Kim, D. Shimizu, A. Osuka, D. Kim
“Effect of bulky meso-substituents on photoinduced twisted intramolecular charge transfer processes in meso-diarylamino subporphyrins”
J. Porphyrins Phthalocyanines 2016, 20, 663-669. [DOI: 10.1142/S1088424616500723]
-
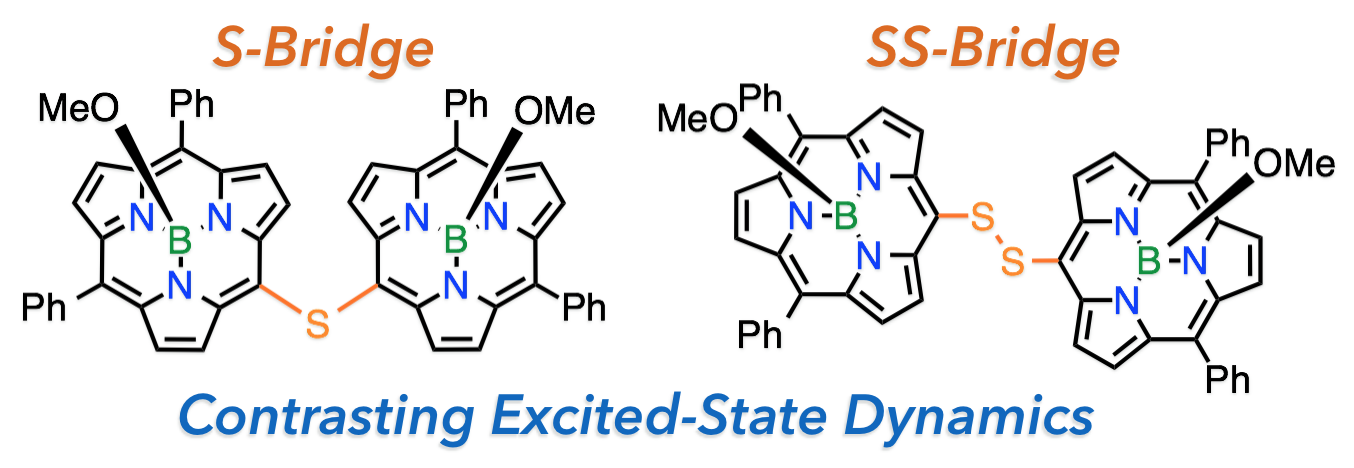
7. G. Copley, D. Shimizu, J. Oh, J. Sung, K. Furukawa, D. Kim, A Osuka
“meso-to-meso Sulfide- and Disulfide-Bridged Subporphyrin Dimers”
Eur. J. Org. Chem. 2016, 1977-1981. [DOI: 10.1002/ejoc.201600285]
-
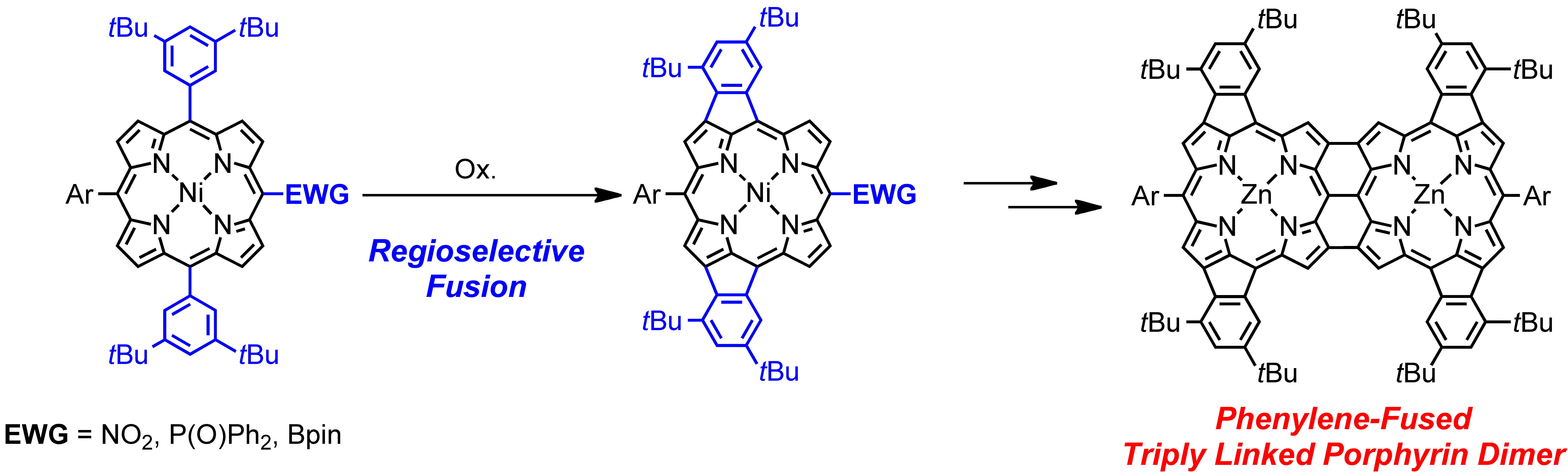
6. N. Fukui, S.-K. Lee, K. Kato, D. Shimizu, T. Tanaka, S. Lee, H. Yorimitsu, D. Kim, A. Osuka
“Regioselective phenylene-fusion reactions of Ni(II)-porphyrins controlled by an electron-withdrawing meso-substituent”
Chem. Sci. 2016, 7, 4059-4066. [DOI: 10.1039/C5SC04748J] (Open access)
-
5. G. Copley, J. Oh, K. Yoshida, D. Shimizu, D. Kim, A. Osuka
“Intramolecular electron transfer reactions in meso-(4-nitrophenyl)-substituted subporphyrins”
Chem. Commun. 2016, 52, 1424-1427. [DOI: 10.1039/c5cc09005a]

-
4. D. Shimizu, J. Oh, K. Furukawa, D. Kim, A. Osuka
“Triarylporphyrin meso-Oxy Radicals: Remarkable Chemical Stabilities and Oxidation to Oxophlorin π-Cations”
J. Am. Chem. Soc. 2015, 137, 15584-15594. [DOI: 10.1021/jacs.5b11223]
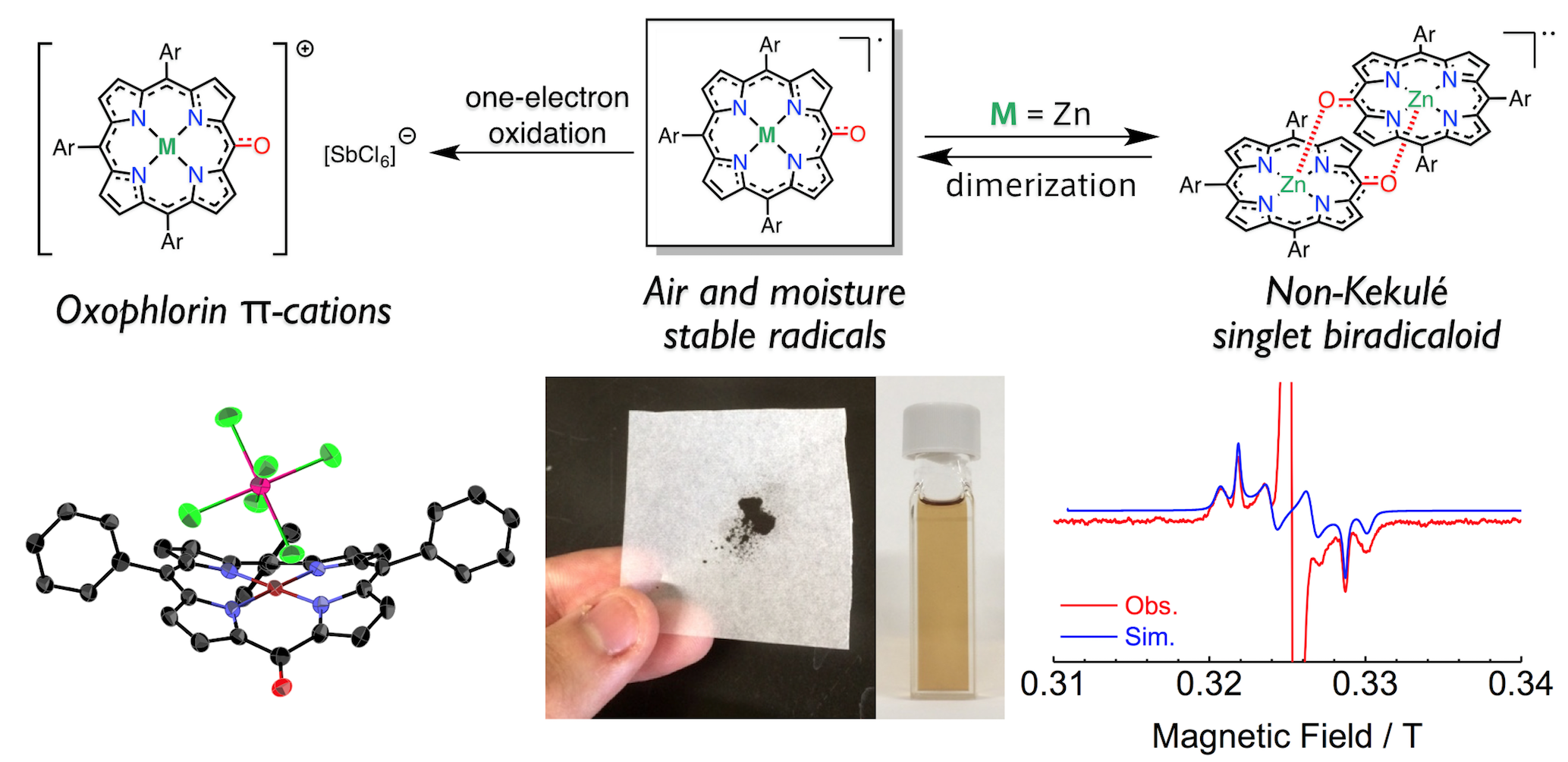
-
3. D. Shimizu, J. Oh, K. Furukawa, D. Kim, A. Osuka
“meso-Hydroxysubporphyrins: a Cyclic Trimeric Assembly and a Stable meso-Oxy Radical”
Angew. Chem. Int. Ed. 2015, 54, 6613-6617. [DOI: 10.1002/anie.201501592]
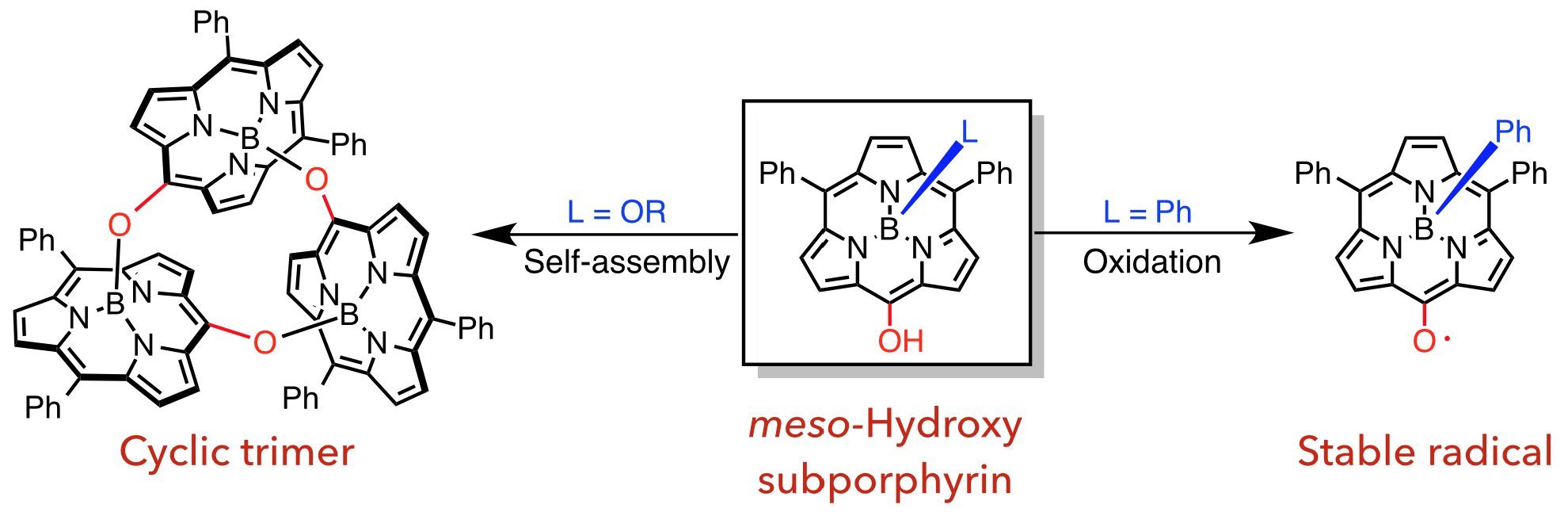
-
2. D. Shimizu, H. Mori, M. Kitano, W.-Y. Cha, J. Oh, T. Tanaka, D. Kim, A. Osuka
“Nucleophilic Aromatic Substitution Reactions of meso-Bromosubporphyrin: Synthesis of a Thiopyrane-Fused Subporphyrin””
Chem. Eur. J. 2014, 20, 16194-16202. [DOI: 10.1002/chem.201405110]
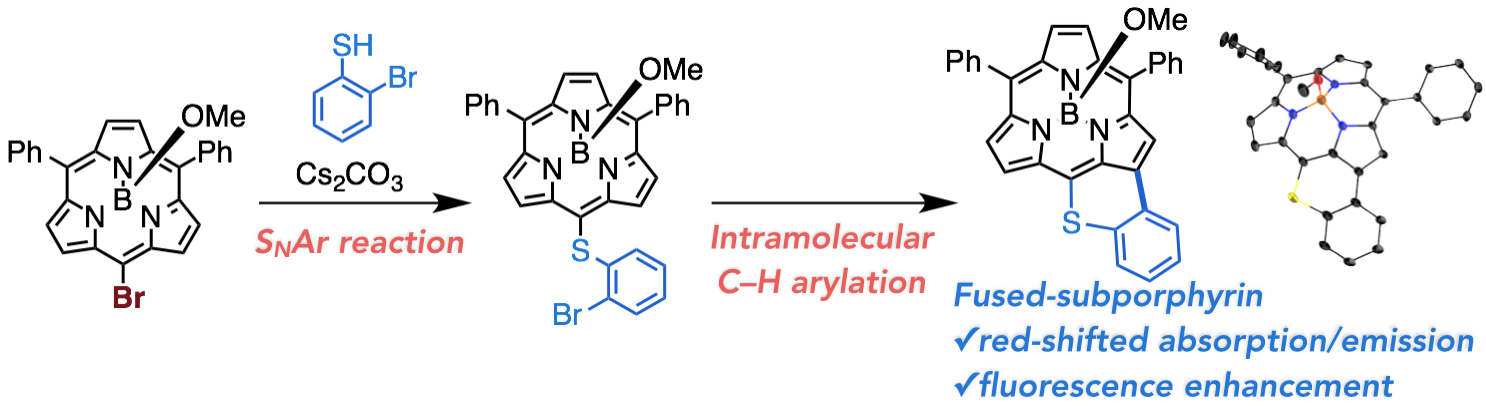
-
1. M. Kitano, D. Shimizu, T. Tanaka, H. Yorimitsu, A. Osuka
“Synthesis of meso-heteroatom-substituted subporphyrins”
J. Porphyrins Phthalocyanines 2014, 18, 659-665. [DOI: 10.1142/s1088424614500394]

Books and Reviews
-
1. D. Shimizu and A. Osuka
“”
Chem. Sci. 2018, 9, 1408−1423. [DOI: 10.1039/C7SC05210C]