Stereoselective Synthesis
We are trying our best to achieve high stereoselectivity in the course of the optimization of the reaction conditions. Not only highly stereoselective processes, but also stereodivergent processes, in which catalysts can control the stereochemical outcome, are highly attractive in that such processes allow synthesis of both stereoisomers with high selectivities.
Stereocomplementary Intramolecular Silaboration
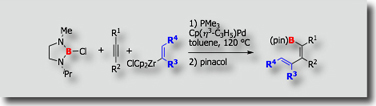
We found some interesting cyclizative borylations, of which stereoselectivities were controlled by the ligand of the catalyst. An example is shown by the platinum-catalyzed intramolecular silaboration of alkenes [Ref.1]. trans-Cyclization products were selectively obtained with a PCyPhSUB{2}; ligand, while cis-products were obtained selectively with a bulky triarylphosphite ligand. One-carbon homologation at the B–C bond followed by oxidation afforded both diastereomers of 1,3,5-triols.
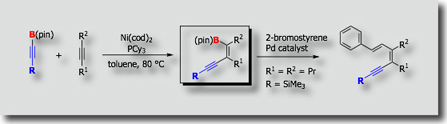
Transmetallative Cyclizative Carboboration
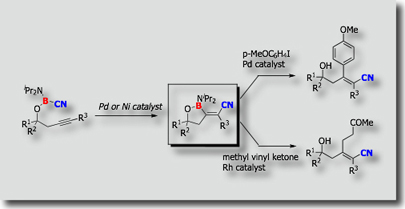
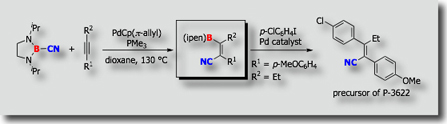
Another example is shown by the palladium-catalyzed cyclizative carboboration reaction [Ref.2]. When PMeSUB{3}; was used as a ligand, cis-addition products were obtained exclusively, while trans-addition products were formed exclusively when PCySUB{3};, P(t-Bu)SUB{3};, or PPhSUB{3}; was utilized as a ligand.
Stereospecific Cationic 1,2-Silyl Shift
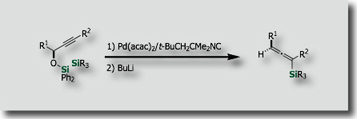
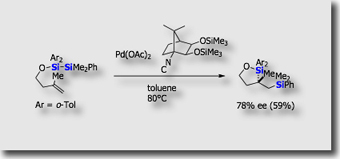
We are also interested in stereoselective or stereospecific conversions of organosilicon compounds for organic synthesis. We for instance established highly stereospecific cationic 1,2-shift of silyl groups in 1,2-siloxetanes synthesized via intramolecular bis-silylation [Ref.3]. This is the first demonstration of cationic syn [1,2] silyl shift that proceeds with the retention of configuration at the migrating terminus.
Related Researches
- Bis-Silylation
- Catalytic Asymmetric Bis-Silylation
- Silaboration
- Silaborative C-C Coupling Reaction
- Intramolecular Silaboration
- Catalytic Asymmetric Silaboration
- Cyanosilylation (Link to references)
- Intramolecular Cyanoboration
- Cyanoboration
- Alkynylboration
- Transmetallative Cyclizative Carboboration
- Transmetallative Three-Component Carboboration
- Asymmetric Polymerization
References
- Ref. 1
- Ligand-Controlled, Complementary Stereoselectivity in the Platinum-Catalyzed Intramolecular Silaboration of Alkenes T. Ohmura, H. Furukawa, M. Suginome, J. Am. Chem. Soc. 2006, 128, 13366-13367, 10.1021/ja065588
- Ref. 2
- Palladium-Catalyzed trans- and cis-Carboboration of Alkynes Tethered to Chloroborane with Organozirconium Reagents: Ligand-Dependent Complementary Stereoselectivity M. Daini, A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2008, 130, 2918-1919, 10.1021/ja711160h
- Ref. 3
- Stereospecific Cationic [1,2]-Silyl Shift with Retention of Configuration at the Migrating Terminus M. Suginome, A. Takama, Y. Ito,J. Am. Chem. Soc. 1998, 120, 1930-1931, 10.1021/ja9737937