B- and Si-Tethered Cyclizations
Intramolecular reactions in which two reactive moieties are connected through removable tethers are attractive strategy in organic synthesis, because higher reactivity and selectivity than the corresponding intermolecular reaction are expected.
Intramolecular Bis-Silylation
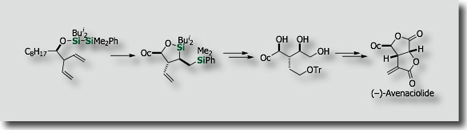
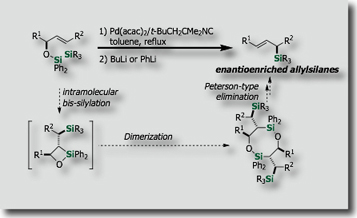
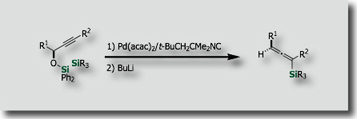
Intramolecular silylations with the tethered silicon atom was particularly attractive. We actually found that intramolecular variants of bis-silylations exhibited high reactivities, higher stereoselectivities, and higher applicability to organic synthesis. Through stereoselective intramolecular bis-silylation of homoallylic alcohols, we made stereoselective total synthesis of (–)-Avenaciolide [Ref. 1]. We also established a synthetic access to highly enantioenriched allylsilanes by intramolecular bis-silylation of allylic alcohols through highly efficient chirality transfer [Ref. 2]. In a similar manner, enantioenriched allenylsilanes are synthesized from enantienriched propargyl alcohols [Ref. 3].
Intramolecular Cyanoboration
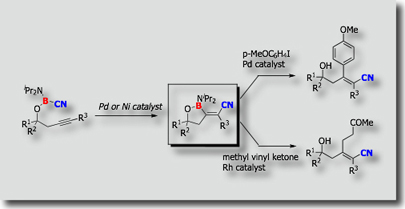
We have also developed intramolecular borylations [Ref. 4]. Intramolecular cyanoboration, for instance, proceeded with high regio- and stereoselectivity than the corresponding intermolecular cyanoboration. Highly substituted a,b-unsaturated nitriles were prepared from the cyanoboration products.
Related Researches
- Transmetallative Cyclizative Carboboration
References
- Ref. 1
- Diastereoselective Intramolecular Bis-Silylation of a Carbon-Carbon Double Bond. A Highly Stereocontrolled Synthesis of (-)-Avenaciolide. M. Suginome, Y. Yamamoto, K. Fujii, Y. Ito, J. Am. Chem. Soc. 1995, 117, 9608–9609, 10.1021/ja00142a047
- Ref. 2
- New Synthesis of (E)-Allylsilanes with High Enantiopurity via Diastereoselective Intramolecular Bis-Silylation of Chiral Allylic Alcohols M. Suginome, A. Matsumoto, Y. Ito, J. Am. Chem. Soc. 1996, 118, 3061–3062, 10.1021/ja954251x
- Synthesis of Highly Enantio-enriched Allylsilanes via Palladium-catalyzed Intramolecular Bis-Silylation. Determination of the Enantiomeric Excesses through Regio- and Stereoselective Hydroboration with 9-BBN M. Suginome, T. Iwanami, A. Matsumoto, Y. Ito, Tetrahedron: Asymmetry, 1997, 8, 859-862, 10.1016/S0957-4166(97)00073-6
- Stereoselective Cyclization of Highly Enantio-Enriched Allylsilanes with Aldehydes via Acetal Formation: New Asymmetric Access to Tetrahydropyrans and Piperidines M. Suginome, T. Iwanami, Y. Ito, J. Org. Chem. 1998, 63, 6096-6097, 10.1021/jo981173y
- Asymmetric Synthesis of 2,3-Disubstituted Oxepanes via Acetalization-Cyclization of an Enantioenriched Functionalized Allylsilane with Aldehydes M. Suginome, T. Iwanami, Y. Ito, Chem. Commun. 1999, 2537-2538, 10.1039/a908603j
- Asymmetric Synthesis of Cyclic Alkenes via Cyclization of Enantioenriched Allylsilanes M. Suginome, T. Iwanami, A. Yamamoto, and Y. Ito, Synlett, 2001, 1042-1045, 10.1055/s-2001-14656
- Solid-Phase Synthesis and Asymmetric Reactions of Polymer-Supported Highly Enantioenriched Allylsilanes M. Suginome, T. Iwanami, and Y. Ito, J. Am. Chem. Soc. 2001, 123, 4356-4357, 10.1021/ja005865r
- Stereoselective Synthesis of Highly Enantioenriched (E)-Allylsilanes by Palladium-Catalyzed Intramolecular Bis-Silylation: 1,3-Chirality Transfer and Enantienrichment via Dimer Formation M. Suginome, T. Iwanami, Y. Ohmori, A. Matsumoto, Y. Ito, Chem. Eur. J., 2005, 11, 2954-2965, 10.1002/chem.200401031
- Ref. 3
- Palladium-Catalyzed Intramolecular Bis–Silylation of Propargylic Alcohols: A New Stereospecific Access to Chiral Allenylsilanes M. Suginome, A. Matsumoto, Y. Ito, J. Org. Chem. 1996, 61, 4884-4885, 10.1021/jo960778w
- Ref. 4
- Palladium- and Nickel-Catalyzed Intramolecular Cyanoboration of Alkynes M. Suginome, A. Yamamoto, M. Murakami, J. Am. Chem. Soc. 2003, 125, 6358-6359, 10.1021/ja0349195
- Synthetic Application of Intramolecular Cyanoboration on the Basis of Removal and Conversion of a Tethering Group by Palladium-Catalyzed Retro-allylation T. Ohmura, T. Awano, M. Suginome, H. Yorimitsu, K. Oshima, Synlett 2008, 423-427, 10.1055/s-2008-1032075