Synthesis and Functions of Helical Polymers のバックアップ(No.1)
- バックアップ一覧
- 差分 を表示
- 現在との差分 を表示
- ソース を表示
- Synthesis and Functions of Helical Polymers へ行く。
- 1 (2009-01-21 (水) 18:12:33)
- 2 (2014-08-18 (月) 15:10:21)
- 3 (2014-08-18 (月) 16:06:27)
Asymmetric Synthesis
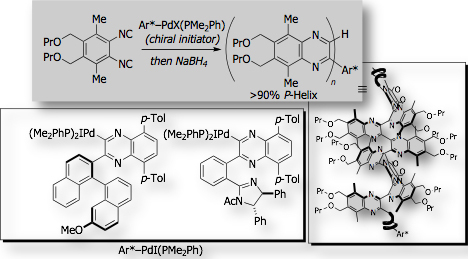
Particular attention is now focused on the exploration of new helical scaffolds that are enantiomerically pure, structurally robust, and readily modifiable at their side chains. Poly(quinoxaline-2,3-diyl)s are quite unique polymer which fulfill all those requirements. However, there was no practical synthetic accesses to single-handed helical poly(quinoxaline)s. We established that living polymerization of 1,2-diisocyanobenzenes with optically active organopalladium initiators could control the screw-sense of the helices with quite high level of screw-sense induction (>90% screw-sense excess) [Ref. 1].
Application to Chiral Polymer Catalysts
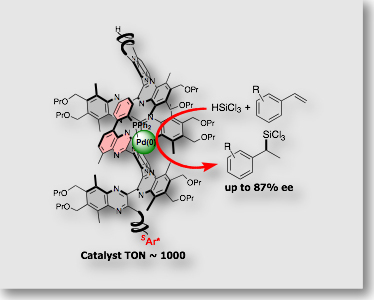
This asymmetric polymerization system was unique in that the optically active group, which remains at the non-living terminus of the polymer chain, controls the helical chirality of the polymer main chain. Since helical conformation of the polymer does not rely on the polymer side chain, but rely on the chiral group at the polymer terminus, the side chains of the polymer is readily modifiable with functional groups, including catalytically active groups. We’ve recently found that polyquinoxaline bearing a coordinating diphenylphosphino group serves as the chiral monodentate ligand in asymmetric hydrosilylation of styrene, affording >85% ee [Ref. 2]. The polymer ligand has a chiral group only at the polymer terminus, which is far away from the reaction sites and thus has no direct participation to the created asymmetric environment around the reaction site.
References
- Ref. 1
- Highly Screw-Sense Selective Polymerization of 1,2-Diisocyano-3,6-di-p-tolylbenzene Initiated by Optically Active Binaphthylpalladium(II) Complexes Y. Ito, T. Ohara, R. Shima, M. Suginome, J. Am. Chem. Soc. 1996, 118, 9188–9189, 10.1021/ja961818g
- Asymmetric Synthesis of Helically Stable Poly(quinoxaline-2,3-diyl)s Having Hydrophilic and/or Hydrophobic Side-chains Y. Ito, T. Miyake, T. Ohara, M. Suginome, Macromolecules 1998, 31, 1697-1699, 10.1021/ma9717855
- Asymmetric Synthesis of Helical Poly(quinoxaline-2,3-diyl)s by Palladium-Mediated Polymerization of 1,2-Diisocyanobenzenes: Effective Control of the Screw-Sense by a Binaphthyl Group at the Chain-End Y. Ito, T. Miyake, S. Hatano, R. Shima, T. Ohara, M. Suginome, J. Am. Chem. Soc. 1998, 120, 11880-11893, 10.1021/ja982500m
- Structural Modification of Living Polymers: Synthesis of Helical Block Copolymers from a Single Monomer via Palladium-Mediated Aromatizing Polymerization of 1,2-Diisocyanobenzenes Y, Ito, T. Miyake, M. Suginome, Macromolecules 2000, 33, 4034-4038, 10.1021/ma991801t
- Highly Effective, Easily Accessible Screw-Sense-Determining End Group in the Asymmetric Polymerization of 1,2-Diisocyanobenzenes M. Suginome, S. Collet, Y. Ito, Org. Lett. 2002, 4, 351-354, 10.1021/ol017113n
- Ref. 2
- Helical Poly(quinoxaline-2,3-diyl)s Bearing Metal-Binding Sites as New Polymer-Based Chiral Ligands T. Yamamoto, M. Suginome, Angew. Chem., Int. Ed. in press, 10.1002/anie.200803719