New Iminium Ion generators
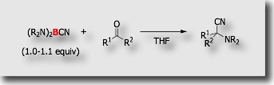
In the course of our study on the synthetic application of cyanoboranes, we found interesting reactivity of bis(dialkylamino)cyanoboranes toward carbonyl compounds. In 1:1 reaction of the cyanoboranes with aldehydes at room temperature, α-dialkylamino nitriles were obtained in high yields [Ref. 1].
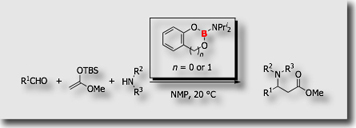
We designed aminoboranes bearing bulky diisopropyl groups on the boron atom. The aminoboranes served as a universal iminium ion generator, allowing use of free sec-amines in the Mannich-type reaction as the source of the amino group through facile amino group exchange on the boron atom [Ref. 2].
The reaction protocol could be extended to other amination reactions, such as reductive amination [Ref. 3] and Ugi reactions [Ref. 4].
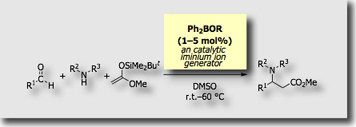
More recently, we found that diphenylborinic acid derivatives serve as “catalytic” iminium ion generators in the Mannich-type reactions [Ref. 5].
References
- Ref. 1
- Bis(dialkylamino)cyanoboranes: highly efficient reagents for the Strecker-type aminative cyanation of aldehydes and ketones. M. Suginome, A. Yamamoto, Y. Ito, Chem. Comm. 2002, 1392-1393, 10.1039/b203645b
- New Look at Boron Enolate Chemistry: Aminative C–C Bond Formation Using Diaminoboron Enolate with Aldehyde M. Suginome, L. Uhelin, A. Yamamoto, M. Murakami, Org. Lett. 2004, 6, 1167-1169, 10.1021/ol0497436
- Ref. 2
- Aminoboranes as "Compatible" Iminium Ion Generators in Aminative C-C Bond Formations M. Suginome, L. Uhelin, M. Murakami, J. Am. Chem. Soc. 2004, 126, 13196-13197, 10.1021/ja045827y
- Aminoboranes as New Iminium Ion Generators in Amination Reactions M. Suginome, Pure Appl. Chem. 2006, 78, 1377-1387, 10.1351/pac200678071377
- Ref. 3
- Reductive Amination of Aldehydes Using Aminoboranes as Iminium Ion Generators M. Suginome, Y. Tanaka, and T. Hasui, Synlett 2006, 1047–1050, 10.1055/s-2006-939070
- Ref. 4
- Acid-free, Aminoborane-mediated Ugi-type Reaction Leading to General Utilization of Secondary Amines Y. Tanaka, Tomoaki Hasui, M. Suginome, Org. Lett. 2007, 9, 4407-4410, 10.1021/ol701570c
- Ref. 5
- Diarylborinic Acid Derivatives as a Catalytic Iminium Ion Generator in the Mannich-type Reaction Using sec-Amines, Aldehydes, and Ketene Silyl Acetals Y. Tanaka, T. Hasui, M. Suginome, Synlett, 2008, 1239–1242, 10.1055/s-2008-1072724