Catalytic Asymmetric Synthesis
Catalytic Asymmetric Silaboration
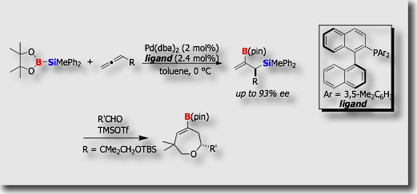
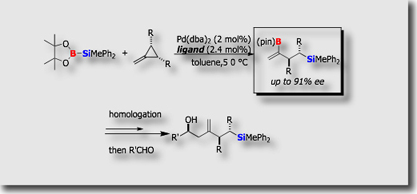
Some new catalytic reactions we established were optimized for application to catalytic asymmetric synthesis. Silaboration of terminal allenes is particularly unique in that allenes undergo the addition reaction at their more substituted C=C bonds, in contrast to many other additions to terminal allenes. This unique aspect of the reaction allowed us to develop its “asymmetric” version, which eventually led to the synthesis of enantioenriched (>90% ee) allylic silanes bearing a boryl group at the β-position [Ref. 1]. Some of the products served as a versatile reagent for the asymmetric synthesis of cyclic alkenylboranes.
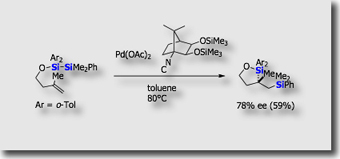
The same catalyst system could be applied to asymmetric silaborative C-C bond cleavage of meso-methylenecyclopropanes [Ref. 2]. As far as we are aware, this reaction was the first catalytic asymmetric C–C bond cleavage reaction which is accompanied by introduction of two functionalities.
Catalytic Asymmetric Bis-Silylation
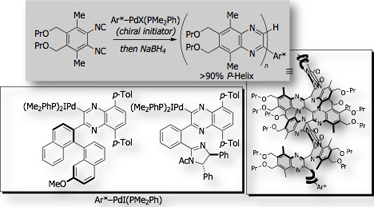
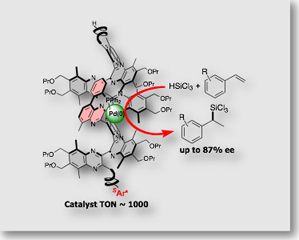
Our research interest has also focused on the exploration of new chiral ligands for organic synthesis. Use of a single-handed helical polymers as a scaffolds for the chiral catalyst is shown in the following section. We also established for the first time that chiral isocyanide serves as chiral spectator ligand for transition metal catalysis [Ref. 3].
Related Research
- Asymmetric Polymerization
References
- Ref. 1
- Enantioface-Selective Palladium-Catalyzed Silaboration of Allenes via Double Asymmetric Induction M. Suginome, T. Ohmura, Y. Miyake, S. Mitani, Y. Ito, M. Murakami, J. Am. Chem. Soc. 2003, 125, 11174-11175, 10.1021/ja0368958
- The Asymmetric Silaboration of Terminal Allenes Bearingα-Stereogenic Centers: Stereoselection Based on “Reagent Control” T. Ohmura, M. Suginome, Org. Lett. 2006, 8, 2503–2506, 10.1021/ol060666j
- Palladium-Catalyzed Asymmetric Silaboration of Allenes T. Ohmura, H. Taniguchi, M. Suginome, J. Am. Chem. Soc. 2006, 128, 13682-13683, 10.1021/ja063934h
- Ref. 2
- Palladium-Catalyzed Asymmetric Silaborative C–C Cleavage of meso-Methylenecyclopropanes T. Ohmura, H. Taniguchi, Yoshiyuki Kondo, M. Suginome, J. Am. Chem. Soc. 2007, 129, 3518-3519, 10.1021/ja0703170
- Ref. 3
- Optically Active Isonitrile Ligand for Palladium-Catalyzed Enantioselective Bis-Silylation of Carbon-Carbon Double Bonds M. Suginome, H. Nakamura, Y. Ito, Tetrahedron Lett. 1997, 38, 555-558, 10.1016/S0040-4039(96)02370-2